Abstract
Twenty-five sophora alkaloids-cinnamic acid hybrids (including matrine-cinnamic acid hybrids, sophoridine-cinnamic acid hybrids, and sophocarpine-cinnamic acid hybrids) were designed, synthesized, and evaluated in vitro against three human tumor cell lines (HeLa, HepG2 and A549) with cisplatin as a positive control. Some matrine-cinnamic acid and sophoridine-cinnamic acid compounds exhibited potent effect against all three cancer cell lines, such as compounds 5b, 5e, 5g, and 6d. The structure-activity relationship study of the synthesized compounds was also performed. Preliminary mechanistic studies indicated that compounds 5e and 6d could induce apoptosis in HepG2 cell line. Further, compounds 5e and 6d altered mitochondrial membrane potential and produced ROS leading to cell apoptosis of HepG2 cells. Overall, our findings suggested that these compounds may provide promising lead compounds for further development as antitumor agents by structural modification.
Keywords: sophora alkaloid, hybrid, cinnamic acid, antitumor, apoptosis
1. Introduction
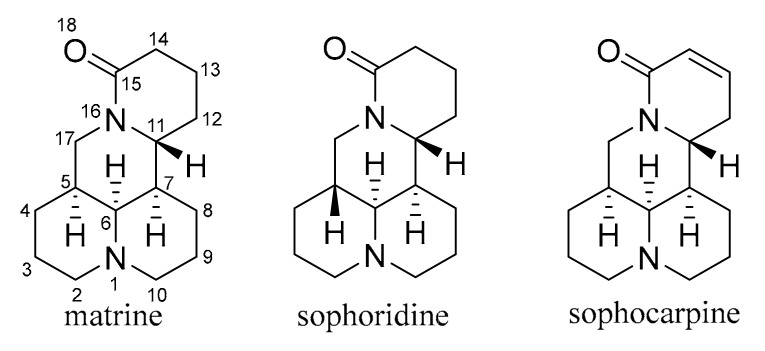
Matrine, sophoridine, and sophocarpine (Figure 1) are the main extractable alkaloid components in the roots of Sophora flavescens Ait (Fabaceae), a common traditional chinese herb. They share the same core structure of quinolizidine and exhibit broad ranging bioactivity profiles that include antitumor [1,2,3,4], anti-inflammatory [5,6,7,8], anti-fibrosis [9], anti-viral [10,11,12], antimicrobial [13], and immunomodulatory effects [14]. It is noteworthy that their antitumor activity has attracted special attention [15,16,17,18,19,20]. Some studies have shown that matrine exerted antitumor effect through inhibiting the proliferation and inducing apoptosis of certain cancer cell lines [21,22,23,24]. Sophoridine, a 5R isomer of matrine, also has antitumor effect and was approved by the Chinese FDA in 2005 as an anticancer drug against malignant trophoblastic tumors [25]. However, the clinical applications of the sophora alkaloids remain limited because of their moderate antitumor activities and short half-life [9,26,27]. As a result, development of their derivatives aiming to improve their therapeutic efficacies is of great importance.
Figure 1.
Structures of matrine, sophoridine, and sophocarpine.
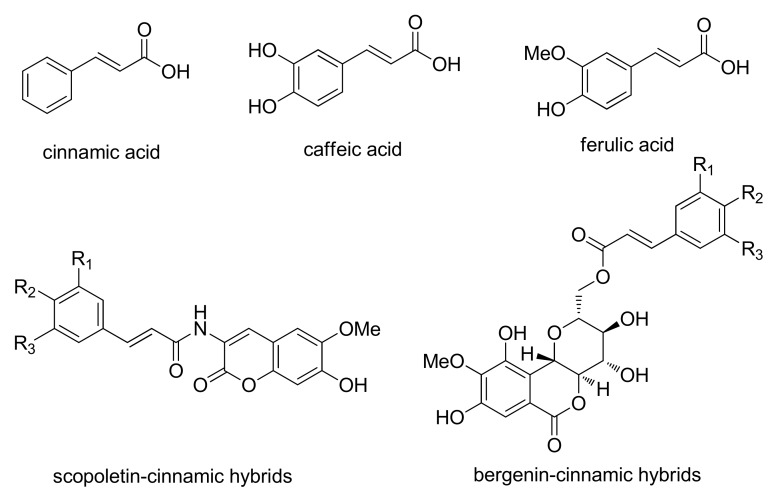
Molecular hybridization is an attractive strategy in drug design by the combination of two active compounds or pharmacophoric units to produce a new hybrid compound with improved affinity and efficacy, when compared to the parent drugs [28]. Additionally, this strategy can result in compounds presenting modified selectivity profile, different and/or dual modes of action and reduced undesired side effects. Cinnamic acid and its analogues, such as caffeic acid and ferulic acid (Figure 2), are naturally occurring aromatic fatty acids which are widely presented in fruits, coffee, and wine [29]. They consist of a common phenyl ring substituted with an acrylic acid group and displayed a variety of biological activities including antitumor effect [30,31,32,33,34]. Owing to their unique structure and impressive biological activity, cinnamoyl moiety was often introduced into many natural and synthetic compounds in the design of antitumor drugs and the synthetic cinnamic acid derivatives could also have an improved anti-cancer activity [35,36,37]. For example, Chen and co-workers synthesized a series of hybrids of scopoletin and substituted cinnamic acid as antitumor agents and the derivatives showed significant enhancement of antitumor activity as compared with the parent compound scopoletin [38]. Mao et al. adopted molecular hybridization strategy to produce bergenin-cinnamic acid hybrids and the evaluation of their antitumor activity also proved that the hybrid compounds were superior to bergenin [39]. These stimulated us to design and synthesize the hybrids of sophora alkaloids and cinnamic acid as antitumor agents by molecular hybridization strategy with improved affinity and efficacy.
Figure 2.
Structures of natural cinnamic acids and the hybrid derivatives of cinnamic acid.
Herein, a series of sophora alkaloids-cinnamic acid hybrids (including matrine-cinnamic acid hybrids, sophoridine-cinnamic acid hybrids, and sophocarpine-cinnamic acid hybrids) were synthesized and their antiproliferative activity was evaluated against a panel of human cell lines using cisplatin as reference. The most promising molecule was selected for further pharmacological mechanism studies.
2. Results and Discussion
2.1. Chemistry
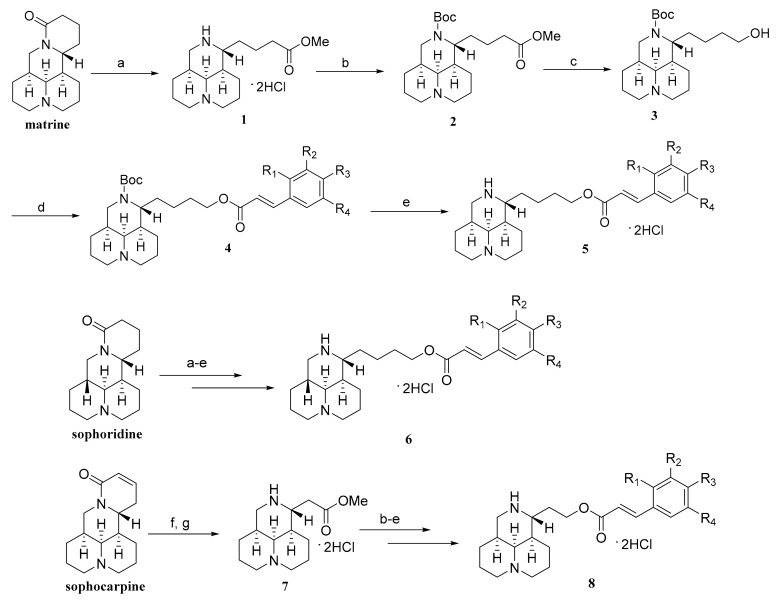
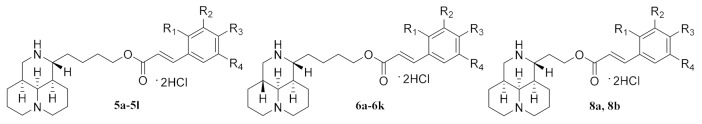
The synthesis of sophora alkaloids-cinnamic derivatives is outlined in Scheme 1. Using commercially available matrine as the starting material, the intermediate methyl matrinic butyrate 1 was obtained through hydrolysis and methyl esterification via hydrochloric acid/MeOH reflux. The Boc protective group was introduced to the 12N-atom of 1 which produced 2 with a yield of 73%, which was used in the following reduction step by LiAlH4 to give 3 [40,41]. Then, the intermediate 3 reacted with substituted trans-cinnamic acid by esterification reaction to afford 4. Finally, deprotection of Boc group by trifluoroacetic acid produced the desired product which was slightly unstable and was further made in the form of hydrochloride salt 5. The hydrochloride salt of sophoridine-cinnamic acid hybrid 6 was obtained from sophoridine as the starting material under the same reaction condition as matrine. For the synthesis of sophocarpine-cinnamic acid hybrids, the key intermediate 7 was acquired via the oxidation reaction from sophocarpine by KMnO4 and 10% H2SO4 followed by an esterification reaction. In the following procedures, the synthetic conditions were also the same as that of matrine and the hydrochloride salt of 8 was acquired. All the structures of sophora alkaloids-cinnamic acid derivatives were shown in Table 1.
Scheme 1.
Reagents and conditions: (a) 2 N HCl, MeOH, reflux; (b) (Boc)2O, K2CO3, CH2Cl2; (c) LiAlH4, THF; (d) substituted trans-cinnamic acid, DMAP, EDCI, CH2Cl2; (e) i) CF3COOH, CH2Cl2, ii) HCl-Et2O; (f) KMnO4, 10%H2SO4; (g) 2 N HCl, MeOH.
Table 1.
The structures of the sophora alkaloid derivatives.
| Compound | Substituents | |||
|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |
| 5a | H | H | H | H |
| 5b | Cl | H | H | H |
| 5c | H | Cl | H | H |
| 5d | H | H | Cl | H |
| 5e | H | Cl | Cl | H |
| 5f | H | Br | H | H |
| 5g | H | H | CF3 | H |
| 5h | H | H | NO2 | H |
| 5i | H | H | CH3 | H |
| 5j | H | H | OCH3 | H |
| 5k | H | OCH2O | H | |
| 5l | H | OCH3 | OCH3 | OCH3 |
| 6a | H | H | H | H |
| 6b | Cl | H | H | H |
| 6c | H | Cl | H | H |
| 6d | H | H | Cl | H |
| 6e | H | Cl | Cl | H |
| 6f | H | Br | H | H |
| 6g | H | H | CF3 | H |
| 6h | H | H | NO2 | H |
| 6i | H | H | CH3 | H |
| 6j | H | H | OCH3 | H |
| 6k | H | OCH2O | H | |
| 8a | Cl | H | H | H |
| 8b | H | H | OCH3 | H |
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity and Structure-Activity Relationships Analysis
All of the synthesized compounds were initially evaluated for their antiproliferative activities against three cancer cell lines (HeLa, HepG2, and A549) with cisplatin as a positive reference using the MTT assay. The results were summarized in Table 2.
Table 2.
Antiproliferative activity of the synthesized derivatives against HeLa, HepG2, and A549 cells.
| Compound | IC50 (μM) a,b | ||
|---|---|---|---|
| HeLa | HepG2 | A549 | |
| 5a | 10.70 ± 1.22 | 14.50 ± 0.37 | 19.73 ± 0.78 |
| 5b | 8.15 ± 0.62 | 7.18 ± 1.99 | 7.30 ± 0.85 |
| 5c | 9.28 ± 1.08 | 10.38 ± 1.38 | 12.17 ± 0.84 |
| 5d | 4.67 ± 0.16 | 8.65 ± 0.77 | 12.69 ± 0.81 |
| 5e | 5.05 ± 0.19 | 6.09 ± 0.54 | 6.65 ± 0.89 |
| 5f | 8.12 ± 0.70 | 7.90 ± 0.66 | 12.31 ± 0.80 |
| 5g | 5.06 ± 0.62 | 8.77 ± 0.96 | 6.54 ± 0.86 |
| 5h | 18.52 ± 0.65 | 20.80 ± 2.82 | 22.42 ± 0.50 |
| 5i | 6.35 ± 0.82 | 9.64 ± 1.92 | 24.40 ± 0.83 |
| 5j | 7.54 ± 0.74 | 15.01 ± 1.27 | 19.21 ± 0.79 |
| 5k | 9.47 ± 0.56 | 11.53 ± 1.21 | 21.50 ± 0.76 |
| 5l | 7.76 ± 0.81 | 8.88 ± 0.78 | 12.24 ± 0.72 |
| 6a | 9.86 ± 0.49 | 11.60 ± 1.29 | >40 |
| 6b | 17.06 ± 0.85 | 7.99 ± 0.84 | 14.16 ± 0.81 |
| 6c | 11.21 ± 0.42 | 6.15 ± 0.56 | 35.88 ± 0.81 |
| 6d | 7.93 ± 0.60 | 6.18 ± 0.45 | 5.47 ± 0.88 |
| 6e | 6.79 ± 1.23 | 3.81 ± 0.24 | 13.48 ± 0.92 |
| 6f | 13.98 ± 0.42 | 4.33 ± 0.37 | 14.48 ± 0.92 |
| 6g | 7.63 ± 0.85 | 5.84 ± 1.18 | >40 |
| 6h | 24.61 ± 4.83 | 19.26 ± 2.54 | >40 |
| 6i | 6.15 ± 0.49 | 4.16 ± 0.29 | 10.63 ± 0.99 |
| 6j | 10.02 ± 3.24 | 11.84 ± 0.95 | >40 |
| 6k | 13.40 ± 0.60 | 12.87 ± 0.78 | 27.27 ± 0.93 |
| 8a | 13.54 ± 2.47 | >40 | >40 |
| 8b | 13.96 ± 0.93 | >40 | >40 |
| matrine | 1883 ± 271 | 1241 ± 225 | >4000 |
| sophoridine | 1532 ± 191 | 1035 ± 167 | >4000 |
| cisplatin | 20.44 ± 6.23 | 39.90 ± 4.31 | 79.20 ± 5.60 |
a IC50 values are shown as mean ± SD from the average of three replicates; b IC50: concentration that causes a 50% reduction of cell growth.
Compared to the parent compound matrine, most compounds exhibited promising antiproliferative activity against one or more cell lines. Among them, compounds 5b, 5e, 5g, and 6d exhibited potent broad-spectrum of activities against all the tested cell lines. It is worth noting that the above four compounds displayed good antiproliferative activity against A549 cell with IC50 values of 7.30 ± 0.85 μM, 6.65 ± 0.89 μM, 6.54 ± 0.86 μM, and 5.47 ± 0.88 μM, respectively, which showed 547 to 731 folds increased activity as compared to matrine.
SAR analysis was first focused on the substituents at the phenyl ring of cinnamoyl moiety of matrine-cinnamic acid hybrids. The result indicated that the introduction of the electron-withdrawing group was beneficial for antitumor activity except nitro substituent (5h). When electron-withdrawing chlorine atom was replaced with a weaker electron-donating methyl, the antitumor activity of 5i slightly decreased against A549 cell line. A similar phenomenon could be found by introduction of other electron-donating groups including MeO-, -OCH2O-, and 3, 4, 5-trimethoxy group (5j–5l). By comparing the substitution position of chlorine, no significant difference was found in the antiproliferative activity, but the substitution at the para-position (5d) seemed to be more favorable than at the ortho- and meta- position (5b and 5c) against HeLa cell line.
To investigate the (R)- or (S)-configuration of the 5-chiral center for the activity, a series of sophoridine (5R isomer of matrine)-cinnamic acid hybrids were prepared. The results showed that most of them displayed equal activities against HeLa and HepG2 cell lines as compared to their corresponding matrine-cinnamic acid hybrids, while some derivatives exhibited decreased activities against A549 cell line compared to their 5S-isomer, such as 6a, 6g, 6h, and 6j. It was noted that the nitro-substituted sophoridine-cinnamic derivative 6h also displayed weak activity which was similar to that of 5h, indicating that the introduction of nitro group was detrimental for activity. To investigate the influence of the chain length to activity, two sophocarpine-cinnamic acid hybrids with shortened side chains were then synthesized and both of them showed weak or no activity against the tested tumor cell lines. The result indicated that the shortening of the butyl chain to ethyl chain was detrimental for activity.
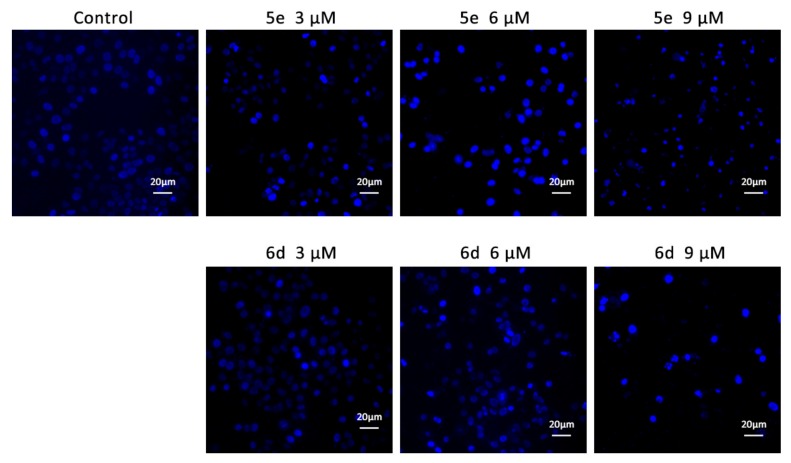
2.2.2. The Morphological Analysis by Hoechst 33258 Staining
The significant antiproliferative activity of these synthetic derivatives encouraged us to further investigate their underlying biological mechanism. As we know, the antiproliferative activity of many anti-cancer agents is closely related to the induction of apoptosis and the progress of apoptosis is often accompanied with morphological changes of chromatin. To examine whether the treatment with these compounds could lead to induce apoptosis, Hoechst 33258 staining was carried out on 5e and 6d treated HepG2 cells. As shown in Figure 3, the control group treated with vehicle solvent displayed uniformly light blue fluorescence, while the cells treated with 5e and 6d (3, 6, and 9 μM) showed sheen blue fluorescence and indicated the typical characteristics of apoptosis including nuclear fragmentation, chromatin condensation, and nuclear shrinkage. This observation revealed that apoptosis induction was involved in 5e and 6d treated HepG2 cells in a dose-dependent manner.
Figure 3.
Hoechst staining for apoptosis. HepG2 Cells were treated with the indicated concentrations of 5e and 6d for 24 h, stained with Hoechst 33258 and examined with a fluorescent microscope.
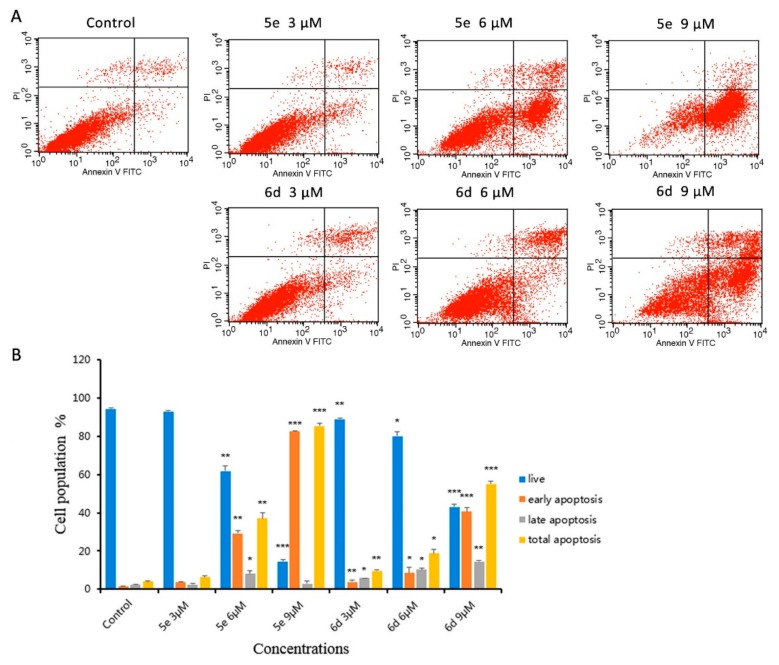
2.2.3. Cell Apoptosis Assay
We further confirmed the apoptotic-inducing effect of 5e and 6d on HepG2 cells through the quantitative analysis of Annexin V-FITC/propidium iodide (PI) staining by flow cytometry. As shown in Figure 4, apoptotic ratio increased accompanied with the increase of concentration of 5e and 6d. The treatment of 5e gave a substantial increase in the total apoptotic ratios from 4.12% of vehicle control to 6.18% (3 μM), 37.13% (6 μM), and 85.34% (9 μM). Under the same conditions, the percentages of total apoptosis of 6d ranged from 9.63% to 55.09%. This result proved that the compounds 5e and 6d induced apoptosis in a dose-dependent manner.
Figure 4.
Apoptosis in HepG2 cells by treatment with 5e and 6d: (A) HepG2 cells were incubated with different concentrations of 5e and 6d for 24 h and the cells were stained with annexin V-FITC and PI, followed by flow cytometry analysis. (B) Quantitative population analysis of annexin V-FITC/PI staining, presented as mean ± SD of three individual experiments, * p < 0.05, ** p < 0.01, *** p < 0.001 vs control group.
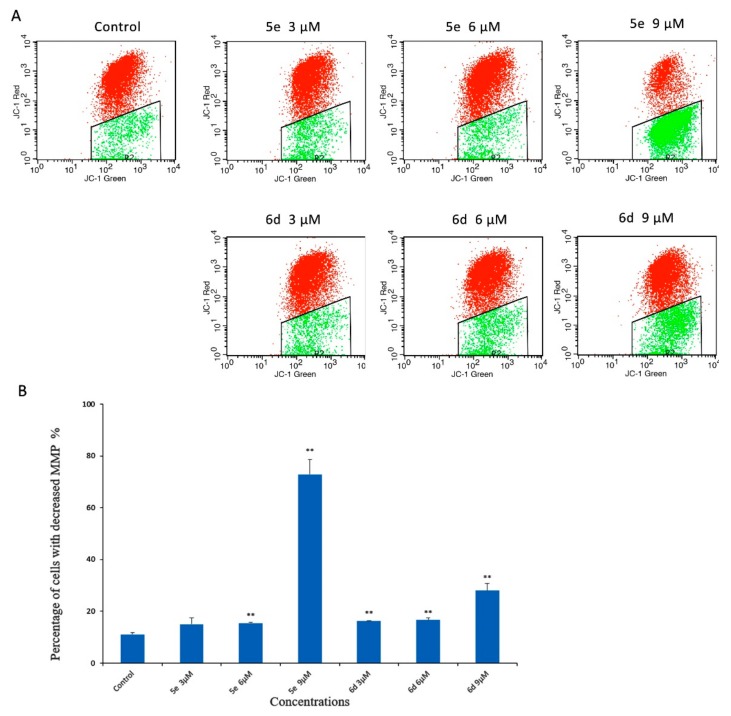
2.2.4. Mitochondria Membrane Potential (MMP) Analysis
The apoptotic pathway of eukaryotic cells included four main pathways: The death receptor-mediated extrinsic pathway, the intrinsic mitochondrial-mediated pathway, granzyme B-mediated pathway and endoplasmic reticulum stress-mediated pathway. The disruption of mitochondrial function is considered as one of the most important apoptotic pathways, which has been recognized as an antitumor target. MMP, tested by JC-1 staining, which is an important parameter reflecting the function of mitochondria. In healthy cells with high mitochondrial membrane potential, JC-1 could spontaneously form complexes and display red fluorescence. On the other hand, in apoptotic or unhealthy cells with low MMP, JC-1 is predominantly a monomer that yields green fluorescence. The effects of 5e and 6d on MMP were further investigated. As shown in Figure 5, the treatment of HepG2 cells with 5e and 6d at different concentrations led to significantly and dose-dependently decrease of MMP. The results suggested that 5e and 6d could induce apoptosis through mitochondrial-mediated apoptotic pathway.
Figure 5.
Effect of 5e and 6d on mitochondrial membrane potential of HepG2 cells. (A) Cells were treated with 5e and 6d for 24 h prior to staining with JC-1 and observed changes in mitochondrial membrane potential by flow cytometry. (B) Quantitative analysis of mitochondrial membrane potential by JC-1 staining, presented as mean ± SD of three individual experiments, * p < 0.05, ** p < 0.01 vs control group.
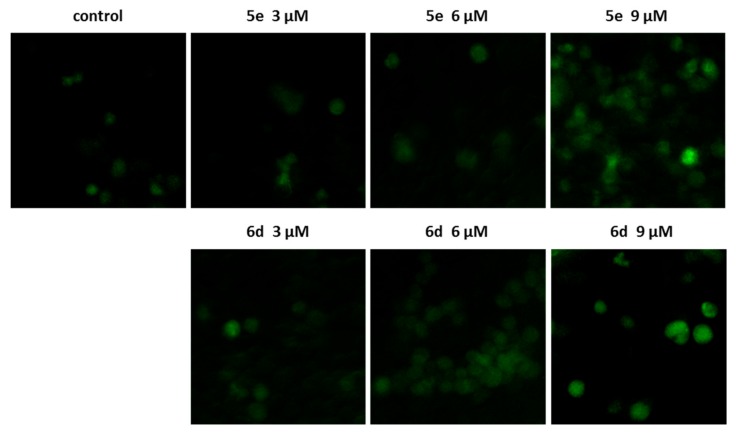
2.2.5. Measurement of Intracellular Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) are important signaling molecules associating with programmed cell death. Excessive ROS accumulation will lead to the change of mitochondrial function and the induction of apoptosis. In order to clarify whether the derivatives could increase cellular ROS levels, levels of intracellular generation of ROS upon treatment with 5e and 6d in HepG2 cells for 24 h were measured by 2, 7-dichlorodihydrofluorescein diacetate (H2DCFDA), a ROS-sensitive fluorescent probe. As seen in Figure 6, both 5e and 6d could effectively promote the production of ROS compared with the control group.
Figure 6.
H2DCFDA staining for ROS induction. HepG2 cells were treated with indicated concentrations of 5e and 6d for 24 h, before staining with 10 μM H2DCFDA for 30 min.
3. Experimental Section
NMR spectra were recorded on Bruker AV III 600 NMR spectrometer instrument (Bruker, Rheinstetten, Germany). Solvent signals (CD3OD: δH = 3.31 ppm/δC = 49.0 ppm) were used as reference. High resolution mass spectra (HRMS) were recorded on a Waters SYNAPT G2 HDMS (Waters, Milford, MA, USA). Reactions were monitored by thin layer chromatography on plates (GF254) supplied by Yantai Chemicals (Yantai, China). Silica gel column chromatography was performed using 200–300 mesh silica gel supplied by Tsingtao Haiyang Chemicals (Tsingtao, China). Unless otherwise noted, all common reagents and solvents were obtained from commercial suppliers (Beijing InnoChem Science & Technology Co., Ltd., Beijing, China) without further purification.
3.1. Chemistry
3.1.1. Methyl Matrinate Dihydrochloride (1)
To a solution of matrine (5.0 g, 20.1 mmol) in MeOH (50 mL) was added 2 mol/L hydrochloric acid (30 mL) and the reaction mixture was heated to reflux for 24 h. Then the solvent was evaporated under vacuum and the residue was triturated with acetone and filtered to afford compound 1 (5.4 g, 76%).
3.1.2. Methyl 12-[(Tert-Butyl)Oxycarbonyl]-Matrinate (2)
To a solution of compound 1 (5.0 g, 14.2 mmol) in MeOH (50 mL) was added (Boc)2O (4.6 g, 21.3 mmol) and anhydrous K2CO3 (5.9 g, 42.6 mmol) at room temperature. After being stirred at room temperature for 4 h, the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2: CH3OH = 40: 1, Rf = 0.3) to give compound 2 (4.8 g, 89%).
3.1.3. 12-[(Tert-Butyl)Oxycarbonyl]-Matrinol (3)
To a solution of compound 2 (4.8 g, 12.6 mmol) in THF (30 mL) was dropwise added a solution of LiAlH4 (574 mg, 15.1 mmol) in THF (5 mL) at 0 °C. After being stirred at room temperature for 6 h, the reaction was quenched with acetone (5 mL) and saturated ammonium chloride solution (5 mL) was added. The mixture was stirred for 30 min and the precipitation was filtered off. The filtrate was concentrated, and the residue was dissolved in ethyl acetate, washed with water and brine, dried with anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (CH2Cl2: CH3OH = 40: 1, Rf = 0.2) to give compound 3 (3.2 g, 72%).
3.1.4. General Procedures for 12-[(Tert-Butyl)Oxycarbonyl]-1′-O-Substituted Cinnamoyl Matrinol (4)
To a solution of compound 3 (200 mg, 0.6 mmol), 4-dimethylaminopyridine (98 mg, 0.8 mmol) and 1-ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride (154 mg, 0.8 mmol) in dry CH2Cl2 (5 mL) was added the corresponding cinnamic acid (0.8 mmol) at room temperature. Upon completion, the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2: CH3OH = 30: 1, Rf = 0.3) to give compound 4.
3.1.5. General Procedures for 1′-O-Substituted Cinnamoyl Matrinol Dihydrochloride (5)
To a solution of compound 4 (0.5 mmol) in CH2Cl2 (3 mL) was added trifluoroacetic acid (0.6 mL) at 0 °C and the reaction mixture was stirred at room temperature. The progress of the reaction was monitored using TLC. After completion of the reaction, the reaction was quenched with saturated NaHCO3 and extracted three times with CH2Cl2. The organic layers were dried with anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue was dissolved in CH2Cl2 and acidified with 2 mol/L HCl-ether (5 mL) to afford target compound 5.
1′-O-cinnamoyl matrinol dihydrochloride (5a) white solid. Yield: 87%; mp: 156.0–157.2 °C; 1H-NMR (600 MHz, CD3OD) δ 7.70 (d, J = 16.0 Hz, 1H), 7.62–7.59 (m, 2H), 7.42–7.38 (m, 3H), 6.54 (d, J = 16.0 Hz, 1H), 4.28–4.23 (m, 2H), 4.11–4.04 (m, 1H), 3.84 (t, J = 13.6 Hz, 1H), 3.70–3.64 (m, 1H), 3.45–3.37 (m, 2H), 3.27 (dd, J = 13.4, 4.6 Hz, 1H), 3.09–3.00 (m, 2H), 2.52–2.44 (m, 1H), 2.26–2.20 (m, 1H), 2.12–1.91 (m, 5H), 1.90–1.74 (m, 6H), 1.73–1.53 (m, 3H); 13C–NMR (150 MHz, CD3OD) δ 168.6, 146.3, 135.7, 131.6, 130.1, 129.3, 118.8, 65.0, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H35N2O2 [M+H]+: 383.2699; found: 383.2691.
1′-O-(2-chloro)cinnamoyl matrinol dihydrochloride (5b) white solid. Yield: 95%. mp: 157.8–158.6 °C; 1H-NMR (600 MHz, CD3OD) δ 8.07 (d, J = 16.0 Hz, 1H), 7.79 (dd, J = 7.7, 1.7 Hz, 1H), 7.47 (dd, J = 7.9, 1.3 Hz, 1H), 7.39 (ddd, J = 7.9, 7.4, 1.7 Hz, 1H), 7.35 (ddd, J = 7.7, 7.4, 1.3 Hz, 1H), 6.58(d, J = 16.0 Hz, 1H), 4.28 (t, J = 6.4 Hz, 2H), 4.07(ddd, J = 12.2, 7.3, 3.4 Hz, 1H), 3.84 (t, J = 13.6 Hz, 1H), 3.69–3.64 (m, 1H), 3.45–3.38 (m, 2H), 3.27 (dd, J = 13.3, 4.6 Hz, 1H), 3.09–3.00 (m, 2H), 2.51–2.44 (m, 1H), 2.25–2.20 (m, 1H), 2.11–1.91 (m, 5H), 1.90–1.75 (m, 6H), 1.74–1.53 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.0, 141.4, 135.8, 133.6, 132.7, 131.2, 129.0, 128.7, 121.8, 65.2, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2302.
1′-O-(3-chloro)cinnamoyl matrinol dihydrochloride (5c) white solid. Yield: 92%. mp: 159.6–160.5 °C; 1H-NMR (600 MHz, CD3OD) δ 7.68–7.63 (m, 2H), 7.55 (ddd, J = 6.3, 2.1, 1.8 Hz, 1H), 7.42–7.37 (m, 2H), 6.58 (d, J = 16.1 Hz, 1H), 4.30–4.22 (m, 2H), 4.08 (ddd, J = 12.2, 7.1, 3.1 Hz, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.69–3.65 (m, 1H), 3.45–3.38 (m, 2H), 3.28 (dd, J = 13.4, 4.7 Hz, 1H), 3.09–3.00 (m, 2H), 2.52–2.46 (m, 1H), 2.26–2.20 (m, 1H), 2.12–1.91 (m, 5H), 1.88–1.75 (m, 6H), 1.74–1.53 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.1, 144.5 137.8, 136.0, 131.6, 131.3, 128.9, 127.6, 120.6, 65.1, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2303.
1′-O-(4-chloro)cinnamoyl matrinol dihydrochloride (5d). white solid. Yield: 91%. mp: 151.3–152.2 °C; 1H-NMR (600 MHz, CD3OD) δ 7.67 (d, J = 16.0 Hz, 1H), 7.61 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 8.5 Hz, 2H), 6.55 (d, J = 16.0 Hz, 1H), 4.30–4.20 (m, 2H), 4.10–4.03 (m, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.68–3.64 (m, 1H), 3.45–3.37 (m, 2H), 3.27 (dd, J = 13.3, 4.4 Hz, 1H), 3.08–3.00 (m, 2H), 2.51–2.45 (m, 1H), 2.25–2.20 (m, 1H), 2.11–1.90 (m, 5H), 1.89–1.75 (m, 6H), 1.74–1.53 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.3, 144.8, 137.3, 134.5, 130.7, 130.2, 119.7, 65.1, 62.7, 56.6, 56.6, 53.6, 43.8, 38.0, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2302.
1′-O-(3, 4-dichloro)cinnamoyl matrinol dihydrochloride (5e). white solid. Yield: 87%. mp: 152.1–153.4 °C; 1H-NMR (600 MHz, CD3OD) δ 7.81 (d, J = 1.5 Hz, 1H), 7.64 (d, J = 16.0 Hz, 1H), 7.58–7.54 (m, 2H), 6.60 (d, J = 16.0 Hz, 1H), 4.30–4.21 (m, 2H), 4.07 (ddd, J = 12.2, 7.1, 3.1 Hz, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.69–3.64 (m, 1H), 3.45–3.38 (m, 2H), 3.28 (dd, J = 13.4, 4.6 Hz, 1H), 3.09–3.00 (m, 2H), 2.51–2.45 (m, 1H), 2.26–2.20 (m, 1H), 2.12–1.89 (m, 5H), 1.88-1.75 (m, 6H), 1.74–1.52 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.0, 143.4, 136.3, 135.0, 134.0, 132.1, 131.0, 128.7, 121.2, 65.2, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H33Cl2N2O2 [M+H]+: 451.1919; found: 451.1918.
1′-O-(3-bromo)cinnamoyl matrinol dihydrochloride (5f) white solid. Yield: 98%. mp: 158.1–159.3 °C; 1H-NMR (600 MHz, CD3OD) δ 7.81–7.79 (m, 1H), 7.65 (d, J = 16.1 Hz, 1H), 7.60 (d, J = 7.7 Hz, 1H), 7.55 (dd, J = 7.9, 1.0 Hz, 1H), 7.33 (dd, J = 7.9, 7.7 Hz, 1H), 6.57 (d, J = 16.1 Hz, 1H), 4.30–4.22 (m, 2H), 4.07 (ddd, J = 12.2, 7.1, 3.2 Hz, 1H), 3.84 (t, J = 13.6 Hz, 1H), 3.68–3.64 (m, 1H), 3.45–3.38 (m, 2H), 3.27 (dd, J = 13.4, 4.7 Hz, 1H), 3.08-3.00 (m, 2H), 2.52–2.44 (m, 1H), 2.25–2.20 (m, 1H), 2.11–1.90 (m, 5H), 1.88–1.74 (m, 6H), 1.73–1.53 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.1, 144.5, 138.1, 134.3, 131.9, 131.8, 128.0, 124.0, 120.6, 65.1, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C24H34BrN2O2 [M+H]+: 461.1804; found: 461.1804.
1′-O-(4-trifluoromethyl)cinnamoyl matrinol dihydrochloride (5g) white solid. Yield: 95%. mp: 155.7–156.8 °C; 1H-NMR (600 MHz, CD3OD) δ 7.81 (d, J = 8.2 Hz, 2H), 7.75 (d, J = 16.1 Hz, 1H), 7.70 (d, J = 8.2 Hz, 2H), 6.68 (d, J = 16.1 Hz, 1H), 4.30–4.25 (m, 2H), 4.08 (ddd, J = 12.2, 7.1, 3.1 Hz, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.69–3.64 (m, 1H), 3.46–3.37 (m, 2H), 3.28 (dd, J = 13.4, 4.7 Hz, 1H), 3.09–2.98 (m, 2H), 2.52–2.45 (m, 1H), 2.26–2.20 (m, 1H), 2.12–1.91 (m, 5H), 1.90–1.75 (m, 6H), 1.74–1.53 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.0, 144.2, 139.5, 132.7 (q, J = 32.4 Hz), 129.7, 126.9 (q, J = 3.7 Hz), 125.4 (q, J = 269.4 Hz), 121.8, 65.2, 62.7, 56.6, 56.6, 53.7, 43.8, 38.0, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C25H34F3N2O2 [M+H]+: 451.2572; found: 451.2568.
1′-O-(4-nitro)cinnamoyl matrinol dihydrochloride (5h) white solid. Yield: 95%. mp: 162.1–163.4 °C; 1H-NMR (600 MHz, CD3OD) δ 8.26 (d, J = 8.8 Hz, 2H), 7.87 (d, J = 8.8 Hz, 2H), 7.77 (d, J = 16.1 Hz, 1H), 6.70 (d, J = 16.1 Hz, 1H), 4.32–4.24 (m, 2H), 4.08 (ddd, J = 12.2, 7.1, 3.1 Hz, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.69–3.63 (m, 1H), 3.47–3.38 (m, 2H), 3.28 (dd, J = 13.3, 4.6 Hz, 1H), 3.09–2.99 (m, 2H), 2.51–2.45 (m, 1H), 2.25–2.19 (m, 1H), 2.12–1.91 (m, 5H), 1.88–1.75 (m, 6H), 1.74–1.55 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 167.7, 150.0, 143.4, 142.0, 130.2, 125.1, 123.3, 65.3, 62.7, 56.7, 56.6, 53.7, 43.8, 38.0, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 21.5, 19.6, 19.5; HRMS m/z calculated for C25H36N3O4 [M+H]+: 428.2549; found: 428.2541.
1′-O-(4-methyl)cinnamoyl matrinol dihydrochloride (5i) white solid. Yield: 94%. mp: 150.8–151.5 °C; 1H-NMR (600 MHz, CD3OD) δ 7.66 (d, J = 16.0 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 6.47 (d, J = 16.0 Hz, 1H), 4.28–4.21 (m, 2H), 4.06 (ddd, J = 12.0, 7.0, 2.7 Hz, 1H), 3.84 (t, J = 13.6 Hz, 1H), 3.67-3.62 (m, 1H), 3.45–3.37 (m, 2H), 3.27 (dd, J = 13.3, 4.4 Hz, 1H), 3.08–2.99 (m, 2H), 2.50–2.42 (m, 1H), 2.35 (s, 3H), 2.44–2.18 (m, 1H), 2.12–1.90 (m, 5H), 1.87–1.75 (m, 6H), 1.73–1.52 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.8, 146.4, 142.3, 132.9, 130.7, 129.3, 117.7, 64.9, 62.7, 56.7, 56.6, 53.6, 43.8, 38.0, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 21.5, 19.6, 19.5; HRMS m/z calculated for C25H37N2O2 [M+H]+: 397.2855; found: 397.2848.
1′-O-(4-methoxy)cinnamoyl matrinol dihydrochloride (5j) white solid. Yield: 90%. mp: 157.6–158.1 °C; 1H-NMR (600 MHz, CD3OD) δ 7.65 (d, J = 16.0 Hz, 1H), 7.55 (d, J = 8.7 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 6.38 (d, J = 16.0 Hz, 1H), 4.27–4.20 (m, 2H), 4.10–4.03 (m, 1H), 3.84 (t, J = 13.6 Hz, 1H), 3.82 (s, 3H), 3.68–3.62 (m, 1H), 3.46–3.38 (m, 2H), 3.27 (dd, J = 13.4, 4.4 Hz, 1H), 3.08–2.98 (m, 2H), 2.49–2.42 (m, 1H), 2.24–2.18 (m, 1H), 2.11–1.90 (m, 5H), 1.86–1.75 (m, 6H), 1.73–1.52 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 169.0, 163.2, 146.2, 131.0, 128.2, 116.0, 115.4, 64.8, 62.7, 56.7, 56.6, 55.9, 53.7, 43.8, 38.0, 32.9, 30.9, 29.6, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C25H37N2O3 [M+H]+: 413.2804; found: 413.2802.
1′-O-(3, 4-methylenedioxy)cinnamoyl matrinol dihydrochloride (5k) white solid. Yield: 84%. mp: 152.3–153.8 °C; 1H-NMR (600 MHz, CD3OD) δ 7.60 (d, J = 15.9 Hz, 1H), 7.16 (d, J = 1.6 Hz, 1H), 7.09 (dd, J = 8.0, 1.6 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.36 (d, J = 15.9 Hz, 1H), 6.00 (s, 2H), 4.26–4.20 (m, 2H), 4.07 (ddd, J = 12.2, 7.2, 3.2 Hz, 1H), 3.85 (t, J = 13.6 Hz, 1H), 3.69–3.64 (m, 1H), 3.45–3.37 (m, 2H), 3.27 (dd, J = 13.3, 4.6 Hz, 1H), 3.09–3.00 (m, 2H), 2.52–2.44 (m, 1H), 2.26–2.20 (m, 1H), 2.12–1.89 (m, 5H), 1.88–1.75 (m, 6H), 1.74–1.52 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.9, 151.3, 149.9, 146.2, 130.1, 125.9, 116.6, 109.5, 107.4, 103.1, 64.9, 62.7, 56.6, 56.6, 53.6, 43.8, 37.9, 32.9, 30.9, 29.5, 25.5, 24.5, 21.9, 19.6, 19.5; HRMS m/z calculated for C25H35N2O4 [M+H]+: 427.2597; found: 427.2590.
1′-O-(3, 4, 5-trimethoxy)cinnamoyl matrinol dihydrochloride (5l) white solid. Yield: 85%. mp: 171.2–172.6 °C; 1H-NMR (600MHz, CD3OD) δ 7.64 (d, J = 15.9 Hz, 1H), 6.94 (s, 2H), 6.50 (d, J = 15.9 Hz, 1H), 4.30–4.20 (m, 2H), 4.12–4.04 (m, 1H), 3.87 (s, 6H), 3.84 (t, J = 13.4 Hz, 1H), 3.78 (s, 3H), 3.68–3.62 (m, 1H), 3.45–3.37 (m, 2H), 3.27 (dd, J = 13.4, 4.1 Hz, 1H), 3.10–2.98 (m, 2H), 2.52–2.44 (m, 1H), 2.27–2.18 (m, 1H), 2.12–1.90 (m, 5H), 1.89–1.74 (m, 6H), 1.73–1.54 (m, 3H); 13C-NMR (150 MHz, CD3OD) δ 168.7, 154.8, 146.4, 141.3, 131.6, 118.2, 106.7, 64.9, 62.7, 61.2, 56.8, 56.7, 56.6, 53.7, 43.8, 38.0, 32.9, 30.9, 29.5, 25.5, 24.5, 22.0, 19.6, 19.5; HRMS m/z calculated for C27H41N2O5 [M+H]+: 473.3015; found: 473.3011.
3.1.6. 1′-O-Substituted Cinnamoyl Sophoridinol Dihydrochloride (6)
The hydrochloride salt of sophoridine-cinnamic acid hybrid 6 was obtained from sophoridine as the starting material and the synthetic route of 6 was the same as that of 5.
1′-O-cinnamoyl sophoridinol dihydrochloride (6a) white solid. Yield: 87%. mp: 171.5–173 °C; 1H-NMR (600 MHz, CD3OD) δ 7.70 (d, J = 16.0 Hz, 1H), 7.65–7.57 (m, 2H), 7.44–7.35 (m, 3H), 6.56 (d, J = 16.0 Hz, 1H), 4.26 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.1, 4.2 Hz, 1H), 3.66–3.61 (m, 1H), 3.53 (dd, J = 8.9, 5.6 Hz, 1H), 3.41–3.32 (m, 2H), 3.24 (dd, J = 12.7, 4.7 Hz, 1H), 3.19–3.13 (m, 1H), 3.02 (t, J = 12.6 Hz, 1H), 2.70–2.60 (m, 1H), 2.45–2.38 (m, 1H), 2.10–1.90 (m, 5H), 1.89–1.76 (m, 5H), 1.73–1.66 (m, 1H), 1.65–1.59 (m, 1H), 1.58–1.50 (m, 1H), 1.49–1.42 (m, 1H); 13C-NMR (150MHz, CD3OD) δ 168.6, 146.3, 135.7, 131.6, 130.1, 129.3, 118.9, 65.1, 59.3, 58.0, 54.1, 46.4, 43.6, 35.8, 29.5, 29.1, 26.9, 26.5, 23.9, 23.8, 22.8, 18.7; HRMS m/z calculated for C24H35N2O2 [M+H]+: 383.2699; found: 383.2699.
1′-O-(2-chloro)cinnamoyl sophoridinol dihydrochloride (6b) white solid. Yield: 92%. mp: 143.0–143.5 °C; 1H-NMR (600 MHz, CD3OD) δ 8.07 (d, J = 16.0 Hz, 1H), 7.81 (dd, J = 7.7, 1.7 Hz, 1H), 7.47 (dd, J = 8.0, 1.1 Hz, 1H), 7.39 (ddd, J = 8.0, 7.4, 1.7 Hz, 1H), 7.35 (ddd, J = 7.7, 7.4, 1.1 Hz, 1H), 6.60 (d, J = 16.0 Hz, 1H), 4.28 (t, J = 6.4 Hz, 2H), 3.84 (dd, J = 12.2, 4.5 Hz, 1H), 3.63 (td, J = 12.9, 3.0 Hz, 1H), 3.53 (dd, J = 9.3, 5.5 Hz, 1H), 3.43–3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.4 Hz, 1H), 3.19–3.13 (m, 1H), 3.03 (t, J = 12.5, 1H), 2.70–2.60 (m, 1H), 2.43 (dt, J = 13.2, 3.8 Hz, 1H), 2.10–1.92 (m, 5H), 1.89–1.78 (m, 5H), 1.73–1.67 (m, 1H), 1.66–1.59 (m, 1H), 1.58–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.1, 141.4, 135.8, 133.6, 132.7, 131.2, 129.0, 128.7, 121.8, 65.3, 59.3, 58.0, 54.1, 46.4, 43.6, 35.8, 29.4, 29.0, 26.9, 26.5, 23.8, 23.7, 22.8, 18.7; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2313.
1′-O-(3-chloro)cinnamoyl sophoridinol dihydrochloride (6c) white solid. Yield: 89%. mp: 135.4–136.8 °C; 1H-NMR (600 MHz, CD3OD) δ 7.67–7.63 (m, 2H), 7.56 (ddd, J = 6.4, 2.4, 1.6 Hz, 1H), 7.41–7.37 (m, 2H), 6.60 (d, J = 16.1 Hz, 1H), 4.26 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.2, 4.4 Hz, 1H), 3.63 (td, J = 12.9, 3.0 Hz, 1H), 3.53 (dd, J = 9.4, 5.5 Hz, 1H), 3.42–3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.4 Hz, 1H), 3.19–3.14 (m, 1H), 3.03 (t, J = 12.7 Hz, 1H), 2.70–2.61 (m, 1H), 2.44 (dt, J = 13.1, 3.8 Hz, 1H), 2.10–1.91 (m, 5H), 1.89–1.77 (m, 5H), 1.72-1.66 (m, 1H), 1.66–1.60 (m, 1H), 1.59–1.51 (m, 1H), 1.50–1.41 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.2, 144.5, 137.8, 136.0, 131.6, 131.2, 128.9, 127.6, 120.7, 65.3, 59.3, 58.0, 54.1, 46.4, 43.6, 35.7, 29.4, 29.1, 26.9, 26.5, 23.8, 23.8, 22.8, 18.7; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2310.
1′-O-(4-chloro)cinnamoyl sophoridinol dihydrochloride (6d). white solid. Yield: 88%. mp: 147.5–148.8 °C; 1H-NMR (600 MHz, CD3OD) δ 7.67 (d, J = 16.0 Hz, 1H), 7.62 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 8.5 Hz, 2H), 6.57 (d, J = 16.0 Hz, 1H), 4.26 (t, J = 6.4 Hz, 2H), 3.82 (dd, J = 12.1, 4.1 Hz, 1H), 3.62 (td, J = 12.7, 2.5 Hz, 1H), 3.55–3.50 (m, 1H), 3.42-3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.1 Hz, 1H), 3.20–3.13 (m, 1H), 3.02 (t, J = 12.7 Hz, 1H), 2.69–2.58 (m, 1H), 2.45–2.36 (m, 1H), 2.15–1.90 (m, 5H), 1.88–1.75 (m, 5H), 1.73–1.66 (m, 1H), 1.65–1.58 (m, 1H), 1.58–1.50 (m, 1H), 1.50–1.41 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.4, 144.8, 137.3, 134.5, 130.8, 130.3, 119.7, 65.2, 59.3, 58.0, 54.1, 46.4, 43.6, 35.8, 29.5, 29.1, 26.9, 26.5, 23.9, 23.7, 22.8, 18.7; HRMS m/z calculated for C24H34ClN2O2 [M+H]+: 417.2309; found: 417.2310.
1′-O-(3, 4-dichloro)cinnamoyl sophoridinol dihydrochloride (6e). white solid. Yield: 72%. mp: 138.8–139.4 °C; 1H-NMR (600 MHz, CD3OD) δ 7.82 (d, J = 1.8 Hz, 1H), 7.63 (d, J = 16.0 Hz, 1H), 7.59–7.53 (m, 2H), 6.62 (d, J = 16.0 Hz, 1H), 4.26 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.2, 4.4 Hz, 1H), 3.63 (td, J = 12.9, 3.0 Hz, 1H), 3.53 (dd, J = 9.5, 5.5 Hz, 1H), 3.41–3.32 (m, 2H), 3.24 (dd, J = 13.2, 4.4 Hz, 1H), 3.19–3.14 (m, 1H), 3.03 (t, J = 12.5 Hz, 1H), 2.69–2.60 (m, 1H), 2.44 (dt, J = 12.8, 3.9 Hz, 1H), 2.10–1.92 (m, 5H), 1.90–1.76 (m, 5H), 1.72–1.67 (m, 1H), 1.66–1.59 (m, 1H), 1.58–1.51 (m, 1H), 1.50–1.41 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.0, 143.4, 136.3, 135.0, 134.0, 132.1, 131.1, 128.7, 121.3, 65.3, 59.3, 58.0, 54.1, 46.4, 43.6, 35.7, 29.4, 29.1, 26.9, 26.5, 23.9, 23.7, 22.8, 18.7; HRMS m/z calculated for C24H33Cl2N2O2 [M+H]+: 451.1919; found: 451.1927.
1′-O-(3-bromo)cinnamoyl sophoridinol dihydrochloride (6f) white solid. Yield: 79%. mp: 146.0–148.0 °C; 1H-NMR (600 MHz, CD3OD) δ 7.80 (s, 1H), 7.64 (d, J = 16.0 Hz, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.33 (dd, J = 7.9, 7.8 Hz, 1H), 6.59 (d, J = 16.0 Hz, 1H), 4.26 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.1, 2.9 Hz, 1H), 3.63 (td, J = 12.8, 2.5 Hz, 1H), 3.53 (dd, J = 9.3, 5.5 Hz, 1H), 3.41–3.32 (m, 2H), 3.24 (dd, J = 13.2, 4.4 Hz, 1H), 3.20–3.14 (m, 1H), 3.03 (t, J = 12.6 Hz, 1H), 2.70–2.60 (m, 1H), 2.47–2.40 (m, 1H), 2.11–1.92 (m, 5H), 1.90–1.77 (m, 5H), 1.72–1.67 (m, 1H), 1.66–1.60 (m, 1H), 1.59–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150MHz, CD3OD) δ 168.2, 144.4, 138.1, 134.2, 132.0, 131.8, 128.0, 123.9, 120.7, 65.3, 59.3, 58.0, 54.1, 46.4, 43.6, 35.7, 29.4, 29.1, 26.9, 26.5, 23.8, 23.8, 22.8, 18.7; HRMS m/z calculated for C24H34BrN2O2 [M+H]+: 461.1804; found: 461.1813.
1′-O-(4-trifluoromethyl)cinnamoyl sophoridinol dihydrochloride (6g) white solid. Yield: 92%. mp: 131.3–132.0 °C;1H-NMR (600 MHz, CD3OD) δ 7.82 (d, J = 8.3 Hz, 2H), 7.74 (d, J = 16.1 Hz, 1H), 7.70 (d, J = 8.3 Hz, 2H), 6.71 (d, J = 16.1 Hz, 1H), 4.27 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.2, 4.4 Hz, 1H), 3.63 (td, J = 12.9, 3.0 Hz, 1H), 3.54 (dd, J = 9.4, 5.4 Hz, 1H), 3.42-3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.4 Hz, 1H), 3.19–3.13 (m, 1H), 3.03 (t, J = 12.7 Hz, 1H), 2.70–2.60 (m, 1H), 2.45 (dt, J = 13.0, 3.8 Hz, 1H), 2.12–1.93 (m, 5H), 1.89–1.76 (m, 5H), 1.73–1.67 (m, 1H), 1.66–1.60 (m, 1H), 1.59–1.51 (m, 1H), 1.50–1.42 (m, 1H);13C-NMR (150MHz, CD3OD) δ 168.0, 144.2, 139.5, 132.7 (q, J = 32.2 Hz), 129.8, 126.9 (q, J = 3.5 Hz), 125.4 (q, J = 271.5 Hz), 121.9, 65.3, 59.3, 58.0, 54.1, 46.4, 43.6, 35.7, 29.4, 29.1, 26.9, 26.5, 23.8, 23.7, 22.8, 18.7; HRMS m/z calculated for C25H34F3N2O2 [M+H]+: 451.2572; found: 451.2576.
1′-O-(4-nitro)cinnamoyl sophoridinol dihydrochloride (6h) white solid. Yield: 92%. mp: 148.0–150.0 °C; 1H-NMR (600 MHz, CD3OD) δ 8.25 (d, J = 8.7 Hz, 2H), 7.87 (d, J = 8.7 Hz, 2H), 7.75 (d, J = 16.1 Hz, 1H), 6.75 (d, J = 16.1 Hz, 1H), 4.27 (t, J = 6.2 Hz, 2H), 3.83 (dd, J = 12.1, 4.1 Hz, 1H), 3.63 (td, J = 13.0, 3.0 Hz, 1H), 3.54 (dd, J = 9.2, 5.3, 1H), 3.43–3.32 (m, 2H), 3.25 (dd, J = 13.1, 4.3 Hz, 1H), 3.20–3.14 (m, 1H), 3.03 (t, J = 12.6 Hz, 1H), 2.70–2.60 (m, 1H), 2.49–2.42 (m, 1H), 2.10–1.93 (m, 5H), 1.89–1.76 (m, 5H), 1.73–1.67 (m, 1H), 1.66–1.61 (m, 1H), 1.60–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 167.8, 149.9, 143.3, 142.0, 130.2, 125.1, 123.4, 65.4, 59.3, 58.0, 54.1, 46.4, 43.6, 35.7, 29.4, 29.1, 26.9, 26.5, 23.8, 23.7, 22.8, 18.7; HRMS m/z calculated for C24H34N3O4 [M+H]+: 428.2549; found: 428.2547.
1′-O-(4-methyl)cinnamoyl sophoridinol dihydrochloride (6i) white solid. Yield: 90%. mp: 129.7–130.5 °C; 1H-NMR (600 MHz, CD3OD) δ 7.65 (d, J = 16.0 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.22 (d, J = 8.2 Hz, 2H), 6.49 (d, J = 16.0 Hz, 1H), 4.24 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.2, 4.5 Hz, 1H), 3.63 (td, J = 12.9, 3.0 Hz, 1H), 3.53 (dd, J = 9.3, 5.5, 1H), 3.42-3.31 (m, 2H), 3.24 (dd, J = 13.2, 4.4 Hz, 1H), 3.19–3.13 (m, 1H), 3.02 (t, J = 12.7 Hz, 1H), 2.70–2.60 (m, 1H), 2.47–2.40 (m, 1H), 2.35 (s, 3H), 2.10–1.92 (m, 5H), 1.88–1.76 (m, 5H), 1.72–1.66 (m, 1H), 1.66–1.59 (m, 1H), 1.59–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.8, 146.3, 142.2, 132.9, 130.7, 129.3, 117.8, 65.1, 59.3, 58.0, 54.1, 46.4, 43.6, 35.8, 29.5, 29.1, 26.9, 26.5, 23.8, 23.8, 22.8, 21.5, 18.7; HRMS m/z calculated for C25H37N2O2 [M+H]+: 397.2855; found: 397.2856.
1′-O-(4-methoxy)cinnamoyl sophoridinol dihydrochloride (6j) white solid. Yield: 81%. mp: 169.0–170.0 °C; 1H-NMR (600 MHz, CD3OD) δ 7.64 (d, J = 16.0 Hz, 1H), 7.56 (d, J = 8.8 Hz, 2H), 6.95 (d, J = 8.8 Hz, 2H), 6.40 (d, J = 16.0 Hz, 1H), 4.24 (t, J = 6.4 Hz, 2H), 3.85–3.80 (m, 4H), 3.63 (td, J = 12.9, 2.6 Hz, 1H), 3.53 (dd, J = 9.2, 5.5 Hz, 1H), 3.42–3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.4 Hz, 1H), 3.19–3.13 (m, 1H), 3.02 (t, J = 12.7 Hz, 1H), 2.70–2.60 (m, 1H), 2.46–2.39 (m, 1H), 2.10–1.92 (m, 5H), 1.87–1.77 (m, 5H), 1.72–1.66 (m, 1H), 1.65–1.59 (m, 1H), 1.58–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 169.1, 163.2, 146.1, 131.0, 128.2, 116.1, 115.4, 65.0, 59.3, 58.0, 55.9, 54.1, 46.4, 43.6, 35.8, 29.5, 29.1, 26.9, 26.5, 23.8, 23.8, 22.8, 18.7; HRMS m/z calculated for C25H37N2O3 [M+H]+: 413.2804; found: 413.2806.
1′-O-(3, 4-methylenedioxy)cinnamoyl sophoridinol dihydrochloride (6k) white solid. Yield: 95%. mp: 142.7–143.5 °C; 1H-NMR (600 MHz, CD3OD) δ 7.60 (d, J = 15.9 Hz, 1H), 7.17 (d, J = 1.3 Hz, 1H), 7.09 (dd, J = 8.0, 1.3 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.38 (d, J = 15.9 Hz, 1H), 6.00 (s, 2H), 4.23 (t, J = 6.4 Hz, 2H), 3.83 (dd, J = 12.0, 3.8 Hz, 1H), 3.63 (td, J = 12.8, 2.3 Hz, 1H), 3.53 (dd, J = 9.1, 5.4 Hz, 1H), 3.42–3.32 (m, 2H), 3.24 (dd, J = 13.1, 4.2 Hz, 1H), 3.19–3.13 (m, 1H), 3.02 (t, J = 12.6 Hz, 1H), 2.70–2.60 (m, 1H), 2.44–2.40 (m, 1H), 2.12–1.91 (m, 5H), 1.88–1.75 (m, 5H), 1.71–1.67 (m, 1H), 1.66–1.59 (m, 1H), 1.58–1.51 (m, 1H), 1.50–1.42 (m, 1H); 13C-NMR (150 MHz, CD3OD) δ 168.9, 151.3, 149.9, 146.1, 130.1, 125.9, 116.7, 109.5, 107.4, 103.1, 65.0, 59.3, 58.0, 54.1, 46.4, 43.6, 35.8, 29.5, 29.1, 26.9, 26.5, 23.8, 23.8, 22.8, 18.7; HRMS m/z calculated for C25H35N2O4 [M+H]+: 427.2597; found: 427.2599.
3.1.7. Methyl Sophocarpinate Dihydrochloride (7)
To a solution of sophocarpine (2.0 g, 8.1 mmol) in 10% H2SO4 (20 mL) was slowly added KMnO4 (1.9 g, 12.2 mmol) in an ice bath and then the reaction mixture was heated to reflux for 24 h. After completion of the reaction, the solution was cooled to room temperature and CH3OH (200 mL) was added. The mixture was stirred for 10 min and the precipitation was filtered off. The filtrate was concentrated, and the residue was dissolved in CH3OH (20 mL) and 2 mol/L hydrochloric acid (20 mL) was added. The reaction mixture was heated to reflux until TLC analysis showed the reaction was completed. After the reaction mixture was neutralized with ammonia water to pH 6–7, the solvent was evaporated under vacuum and the residue was dissolved in CH3OH (100 mL). The precipitation was filtered off and the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2: CH3OH = 30: 1, Rf = 0.2) to give 7 (1.8 g, 68%)
The hydrochloride salt of sophocarpine-cinnamic acid hybrid 8 was obtained from 7 and the following synthetic route of 8 was the same as that of 5.
1′-O-(2-chloro)cinnamoyl sophocarpinol dihydrochloride (8a) white solid. Yield: 94%. mp: 134.1–135.2 °C; 1H-NMR (600 MHz, CD3OD) δ 8.10 (d, J = 16.0 Hz, 1H), 7.85 (dd, J = 7.7, 1.7 Hz, 1H), 7.46 (d, J = 7.9 Hz, 1H), 7.39 (ddd, J = 7.9, 7.5, 1.7 Hz, 1H), 7.35 (dd, J = 7.7, 7.5 Hz, 1H), 6.75 (d, J = 16.0 Hz, 1H), 4.45–4.40 (m, 2H), 4.32–4.25 (m, 1H), 3.90 (t, J = 13.6 Hz, 1H), 3.74–3.68 (m, 1H), 3.47–3.39 (m, 2H), 3.33 (dd, J = 13.4, 4.6 Hz, 1H), 3.11–3.01 (m, 2H), 2.58–2.49 (m, 1H), 2.45–2.38 (m, 1H), 2.36–2.28 (m, 1H), 2.16 (d, J = 14.6 Hz, 1H), 2.09–1.91 (m, 4H), 1.91–1.74 (m, 4H); 13C-NMR (150 MHz, CD3OD) δ 167.7, 141.7, 135.8, 133.6, 132.7, 131.2, 129.2, 128.6, 121.7, 62.6, 61.2, 56.6, 56.6, 51.8, 43.9, 38.1, 32.8, 30.6, 25.5, 24.6, 19.6, 19.4; HRMS m/z calculated for C22H30ClN2O2 [M+H]+: 389.1996; found: 389.1989.
1′-O-(4-methoxy)cinnamoyl sophocarpinol dihydrochloride (8b) white solid. Yield: 90%. mp: 145.4–146.5 °C; 1H-NMR (600 MHz, CD3OD) δ 7.76 (d, J = 16.0 Hz, 1H), 7.62 (d, J = 8.8 Hz, 2H), 6.95 (d, J = 8.8 Hz, 2H), 6.50 (d, J = 16.0 Hz, 1H), 4.42 (dt, J = 11.9, 5.5 Hz, 1H), 4.39–4.32 (m, 1H), 4.29 (ddd, J = 12.5, 8.0, 2.9 Hz, 1H), 3.91 (t, J = 13.7 Hz, 1H), 3.82 (s, 3H), 3.71–3.68 (m, 1H), 3.47–3.39 (m, 2H), 3.32 (dd, J = 13.4, 4.6 Hz, 1H), 3.11–3.00 (m, 2H), 2.57–2.50 (m, 1H), 2.44–2.36 (m, 1H), 2.35–2.28 (m, 1H), 2.14 (d, J = 11.5 Hz, 1H), 2.10–1.91 (m, 4H), 1.91–1.75 (m, 4H); 13C-NMR (150 MHz, CD3OD) δ 168.6, 163.3, 146.7, 131.2, 128.3, 115.8, 115.4, 62.6, 60.8, 56.7, 56.6, 55.9, 51.8, 43.9, 38.2, 32.8, 30.8, 25.5, 24.6, 19.6, 19.4; HRMS m/z calculated for C23H33N2O3 [M+H]+: 385.2491; found: 385.2490.
The NMR spectra of the compounds 5a–5l, 6a–6k and 8a-8b are shown in the electronic Supplementary Materials.
3.2. Biological Evaluation
3.2.1. Cell Lines and Cell Culture
The cell lines, Cervical (HeLa), Lung (A549) and Liver (HepG2) which were used in this study were procured from National Infrastructure of Cell Line Resource and were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were maintained in a humidified incubator with 95% air and 5% CO2 at 37 °C.
3.2.2. Antiproliferative Activity
The antiproliferative activity of the synthetic compounds was evaluated by the MTT assay in vitro, with cisplatin as a positive control. In brief, the cells were seeded into 96-well plates at the density of 5 × 103 cells/well and were cultured overnight for adherence. The test compounds were prepared from 4 mM or 16 mM stock solution in DMSO according to preliminary screening results. Then these stocks solution were serially diluted with DMEM medium with the final DMSO concentration no greater than 0.5%. The concentration was set to 20 μM, 10 μM, 5 μM, 2.5 μM, and 1.25 μM for potent compounds and 80 μM, 40 μM, 20 μM, 10 μM, and 5 μM for less potent compounds. The cells were treated with different concentrations of test compounds for 48 h. After that, 20 μL MTT (5 mg/mL) were added to each well and the plates were further incubated for 4 h. The medium was carefully removed and the formazan crystals were dissolved in 150 μL DMSO. The absorbance at 490 nm was measured on a microplate reader (TECAN Infinite M1000, Austria). The cell viability was determined in terms of IC50 value.
3.2.3. Hoechst 333258 Staining
HepG2 cells were seeded at a density of 2 × 105 cells/well on coverslips in 6-well plates and allowed to attach overnight, the media was replaced with fresh media containing with vehicle or compounds 5e and 6d at a dose of 3, 6, and 9 μM, respectively and further incubated for 24 h. The cells were washed with PBS twice and stained with Hoechst 333258 (5 μg/mL) for 15 min at 37 °C. After washing the coverslips twice with PBS, the cells were visualized with a fluorescence microscopy (Zeiss, Germany) with a DAPI filter.
3.2.4. Cell Apoptosis Assay
Apoptosis was detected by flow cytometry using Annexin V-FITC/Propidium Iodide (PI) double staining assay. Compounds 5e and 6d were chosen for cell apoptosis assay, and the concentration was set to 3 μM, 6 μM, and 9 μM by diluting the 4M stock solution. The final concentrations of DMSO were 0.075%, 0.15%, and 0.225%, respectively. HepG2 cells (2 × 105 cells/well) were cultured in 6-well plates and allowed to attach overnight. After treatment with compounds 5e and 6d as described in Hoechst 333258 staining, the cells were harvested by trypsinization. According to manufacturer’s instructions of the kit (Miltenyi Biotec GmbH), resuspend the cells in 100 μL binding buffer and incubate the cell suspension with 10 μL FITC-Annexin V for 15 min at room temperature in the dark. Wash cells with binding buffer. Resuspend cell pellet in 500 μL binding buffer with 5 μL PI solution. Cellular fluorescence was measured by flow cytometry analysis (FACS CaliburTM, BD Biosciences, CA, USA).
3.2.5. Mitochondrial Membrane Potential Assay
The percentage of decreased MMP of cells was measured using a JC-1 mitochondrial membrane potential detection kit (Beyotime, China) according to the manufacturer’s instructions. HepG2 cells were seeded at a density of 2 × 105 cells/well in 6-well plates and incubated overnight. After treatment with compounds 5e and 6d as described in Hoechst 33258 staining, the cells were collected by trypsinization. Resuspend the cells in 0.5 mL cell culture medium, add 0.5 mL of freshly prepared JC-1 working solution and incubate for 20 min at 37 °C. in the dark. Then the cells were washed twice with staining buffer without JC-1 and subjected to flow cytometry (FACS CaliburTM, BD Biosciences, CA, USA).
3.2.6. Measurement of Intracellular ROS Level
The fluorescent imaging of ROS generation was performed on HepG2 cells. HepG2 cells was seeded at 1 × 105 cells/well in 6-well plate and were incubated at 37 °C under a 5% CO2 atmosphere for 24 h before treated with compounds 5e and 6d at the indicated time and dosages. The cells were then incubated with 10 μM H2DCFDA for 30 min, washed with PBS for twice and observed under a Zeiss Axio Observer 3 fluorescence microscope.
3.2.7. Statistical Analysis
All data were expressed as mean ± SD from at least three independent experiments. SPSS 17.0 software was used for statistical analysis. Student’s t-test was performed. p-value < 0.05 was considered statistically significant.
4. Conclusions
A series of hybrids of sophora alkaloids and substituted cinnamic acids were designed, synthesized and evaluated in vitro against three human tumor cell lines. Some of the synthesized compounds displayed promising activities against all the tested cell lines. The structure-activity relationship study showed that the introduction of electron-withdrawing groups to the benzene ring was beneficial for the antitumor activity and shortening of the butyl chain of matrinol to ethyl chain was detrimental for activity. The mechanistic studies indicated that 5e and 6d could induce mitochondrial-mediated apoptosis in HepG2 cells. All considered, our studies suggested that these compounds may provide potential lead compounds for further development as cancer chemotherapeutics.
Supplementary Materials
The following are available online. 1H-NMR and 13C-NMR spectra of the synthesized compounds.
Author Contributions
Conceptualization, H.S. and Z.Z.; methodology, L.M. and H.J.; formal analysis, M.Y.; investigation, H.S., L.L. and Y.T.; writing—original draft preparation, H.S. and Y.T.; writing—review and editing, L.L. and T.Z.; supervision, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (81502929); the Drug Innovation Major Project (2018ZX09711-001-005-008); CAMS Innovation Fund for Medical Sciences (2016-I2M-3-015) and Beijing Natural Science Foundation (7192129).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 5a-5l, 6a-6k and 8a-8b are available from the authors.
References
- 1.Zhang Y., Zhang H., Yu P.F., Liu Q., Liu K., Duan H.Y., Luan G.L., Yagasaki K., Zhang G.Y. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu P.F., Liu Q., Liu K., Yagasaki K., Wu E., Zhang G.Y. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X.Y., Fang H., Yang Z.G., Wang X.Y., Ruan L.M., Fang D.R., Ding Y.G., Wang Y.N., Zhang Y., Jiang X.L., et al. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int. J. Dermatol. 2008;47:448–456. doi: 10.1111/j.1365-4632.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 4.Li M.C., Wang Y.L., Dong L.Y. Effect and mechanism of allomatrine in proliferation and invasion in vitro inhibition of human lung cancer A549 cell line. Chin. Pharm. J. 2015;50:1111–1116. [Google Scholar]
- 5.Hu H.G., Wang S.Z., Zhang C.M., Wang L., Ding L., Zhang J.P., Wu Q.Y. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorgan. Med. Chem. Lett. 2010;20:7537–7539. doi: 10.1016/j.bmcl.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 6.Huang W.C., Chan C.C., Wu S.J., Chen L.C., Shen J.J., Kuo M.L., Chen M.C., Liou C.J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014;151:470–477. doi: 10.1016/j.jep.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 7.Sun D.Q., Wang J., Yang N.D., Ma H.X. Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-κB signaling in airway epithelial cells and asthmatic mice. Biochem. Biophys. Res. Commun. 2016;477:83–90. doi: 10.1016/j.bbrc.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Liu J.Y., Hu J.H., Zhu Q.G., Li F.Q., Wang J., Sun H.J. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int. Immunopharmacol. 2007;7:816–823. doi: 10.1016/j.intimp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J.P., Zhang M., Zhou J.P., Liu F.T., Zhou B., Xie W.F., Guo C., Zhang C., Qian D.H. Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol. Sin. 2001;22:183–186. [PubMed] [Google Scholar]
- 10.Yang Y.J., Xiu J.H., Zhang X., Zhang L.F., Yan K., Qin C., Liu J.N. Antiviral effect of matrine against human enterovirus 71. Molecules. 2012;17:10370–10376. doi: 10.3390/molecules170910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.Y., Zhu H.Y., Ye G., Huang C.G., Yang Y.Z., Chen R.Z., Yu Y., Cui X.L. Antiviral effects of sophoridine against coxsackievirus B3 and its pharmacokinetics in rats. Life Sci. 2006;78:1998–2005. doi: 10.1016/j.lfs.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Guo B., Li C., Deng Z.P., Chen S.X., Ji Z.H., Zhang J.W., Chen M.F., Xu F. A new method for measurement of (−)-sophocarpine, a candidate therapeutic for viral myocarditis, in plasma: Application to a toxicokinetic study in beagle dogs. Rapid Commun. Mass Spectrom. 2005;19:2840–2848. doi: 10.1002/rcm.2132. [DOI] [PubMed] [Google Scholar]
- 13.Küçükboyaci N., Özkan S., Adigüzel N., Tosun F. Characterisation and antimicrobial activity of Sophora alopecuroides L. var. alopecuroides alkaloid extracts. Turk. J. Biol. 2011;35:379–385. [Google Scholar]
- 14.Liu X.Y., Wang R.T., Cui C., Wang Z.H., Tong H., Deng Y.Q., Zhang Z.Z. Effect of oxymatrine on L929 tumor cell-induced immunosuppression. Chin. J. Immunol. 2009;3:216–220. [Google Scholar]
- 15.Li H.J., Li X.J., Bai M.L., Suo Y.E., Zhang G.H., Cao X.Y. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int. J. Exp. Pathol. 2015;8:14793–14799. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M., Li J.Q., Li T., Chen G.S., Zhang Z.Q., Si Z.Z. Matrine induced apoptosis in Hep3B cells-via the inhibition of MDM2. Mol. Med. Rep. 2017;15:442–450. doi: 10.3892/mmr.2016.5999. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Liu Y.H., Jiang J.S., Cui H.B. Antitumor effects of matrine on cancer stem like cells isolated from the human liver cancer SMMC-7721 cell line. Oncol. Lett. 2018;15:1777–1782. doi: 10.3892/ol.2017.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J.L., Hu S.P., Wang W.Y., Li Y.M., Zhi W.L., Lu S., Shi Q., Wang Y.J., Yang Y.P. Matrine inhibits prostate cancer via activation of the unfolded protein response/endoplasmic reticulum stress signaling and reversal of epithelial to mesenchymal transition. Mol. Med. Rep. 2018;18:945–957. doi: 10.3892/mmr.2018.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z.H., Zhang F., Bai C., Yao C., Zhong H.R., Zou C.P., Chen X. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2017;36:124–134. doi: 10.1186/s13046-017-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X.Y., Zeng C., Li J.G. Study of pharmacokinetic and anti-tumor effect of sophocarpine. Tradit. Chin. Drug Res. Clin. Pharmacol. 2017;28:644–648. [Google Scholar]
- 21.Dai Z.J., Gao J., Ji Z.Z., Wang X.J., Ren H.T., Liu X.X., Wu W.Y., Kang H.F., Guan H.T. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J. Ethnopharmacol. 2009;123:91–96. doi: 10.1016/j.jep.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P., Wang Z.M., Chong T., Ji Z.Z. Matrine inhibits proliferation and induces apoptosis of the androgen-independent prostate cancer cell line PC-3. Mol. Med. Rep. 2012;5:783–787. doi: 10.3892/mmr.2011.701. [DOI] [PubMed] [Google Scholar]
- 23.Liang C.Z., Zhang J.K., Shi Z.L., Liu B., Shen C.Q., Tao H.M. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother. Pharmacol. 2012;69:317–331. doi: 10.1007/s00280-011-1699-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S.H., Zhang Y., Zhuang Y., Wang J.J., Ye J.Q., Zhang S., Wu J.B., Yu K., Han Y.X. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS ONE. 2012;7:e46853. doi: 10.1371/journal.pone.0046853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X.M., Wu Y.G., Pan D.X., Wu L.K., Yu Y.H., Zhang A.H., Chen S.L., Guan Z.Z., Yang X.Y. Sophoridine is a new antitumor medicine with new molecular structure. Chin. J. New Drugs. 2006;15:654–657. [Google Scholar]
- 26.Yong J.P., Wu X.Y., Lu C.Z. Anticancer advances of matrine and its derivatives. Curr. Pharm. Des. 2015;21:3673–3680. doi: 10.2174/1381612821666150122123748. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L., Liu W.T., Zhang R.W., Wang Z.W., Shen Z.D., Chen X.H., Bi K.S. Pharmacokinetic study of matrine, oxymatrine and oxysophocarpine in rat plasma after oral administration of Sophora flavescens Ait. extract by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008;47:892–898. doi: 10.1016/j.jpba.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Claudio V.-J., Amanda D., Vanderlan da Silva B., Eliezer J.B., Carlos Alberto Manssour F. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14:1829–1852. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 29.Hoskins J.A. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J. Appl. Toxicol. 1984;4:283–292. doi: 10.1002/jat.2550040602. [DOI] [PubMed] [Google Scholar]
- 30.Lapeyre C., Delomenède M., Bedos-Belval F., Duran H., Nègre-Salvayre A., Baltas M. Design, synthesis, and evaluation of pharmacological properties of cinnamic derivatives as antiatherogenic agents. J. Med. Chem. 2005;48:8115–8124. doi: 10.1021/jm050454c. [DOI] [PubMed] [Google Scholar]
- 31.Gaspar A., Garrido E.M., Esteves M., Quezada E., Milhazes N., Garrido J., Borges F. New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. Eur. J. Med. Chem. 2009;44:2092–2099. doi: 10.1016/j.ejmech.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Menezes J.C.J.M.D.S., Kamat S.P., Cavaleiro J.A.S., Gaspar A., Garrido J., Borges F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur. J. Med. Chem. 2011;46:773–777. doi: 10.1016/j.ejmech.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Yen G.C., Chen Y.L., Sun F.M., Chiang Y.L., Lu S.H., Weng C.J. A comparative study on the effectiveness of cis- and trans-form of cinnamic acid treatments for inhibiting invasive activity of human lung adenocarcinoma cells. Eur. J. Pharm. Sci. 2011;44:281–287. doi: 10.1016/j.ejps.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Liu L., Hudgins W.R., Shack S., Yin M.Q., Samid D. Cinnamic acid: A natural product with potential use in cancer intervention. Int. J. Cancer. 1995;62:345–350. doi: 10.1002/ijc.2910620319. [DOI] [PubMed] [Google Scholar]
- 35.De P., Baltas M., Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-A review. Curr. Med. Chem. 2011;18:1672–1703. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]
- 36.Zhou T., Shi Q., Bastow K.F., Lee K.H. Antitumor agents 286. Design, synthesis, and structure−activity relationships of 3′R,4′R-disubstituted-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DSP) analogues as potent chemosensitizers to overcome multidrug resistance. J. Med. Chem. 2010;53:8700–8708. doi: 10.1021/jm101249z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian Y., Zhang H.J., Zhang H., Xu C., Zhao J., Zhu H.L. Synthesis, molecular modeling, and biological evaluation of cinnamic acid metronidazole ester derivatives as novel anticancer agents. Bioorgan. Med. Chem. 2010;18:4991–4996. doi: 10.1016/j.bmc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Li L.H., Zhao P., Hu J.L., Liu J.H., Liu Y., Wang Z.Q., Xia Y.F., Dai Y., Chen L. Synthesis, in vitro and in vivo antitumor activity of scopoletin-cinnamic acid hybrids. Eur. J. Med. Chem. 2015;93:300–307. doi: 10.1016/j.ejmech.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Liang C.Y., Pei S.M., Ju W.H., Jia M.Y., Tian D.N., Tang Y.H., Mao G.N. Synthesis and in vitro and in vivo antitumour activity study of 11-hydroxyl esterified bergenin/cinnamic acid hybrids. Eur. J. Med. Chem. 2017;133:319–328. doi: 10.1016/j.ejmech.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Wang S.G., Kong L.Y., Li Y.H., Cheng X.Y., Su F., Tang S., Bi C.W., Jiang J.D., Li Y.H., Song D.Q. Structure–activity relationship of N-benzenesulfonyl matrinic acid derivatives as a novel class of coxsackievirus B3 inhibitors. Bioorgan. Med. Chem. Lett. 2015;25:3690–3693. doi: 10.1016/j.bmcl.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Cheng X.Y., Li Y.H., Tang S., Zhang X., Wang Y.X., Wang S.G., Jiang J.D., Li Y.H., Song D.Q. Synthesis and evaluation of halogenated 12N-sulfonyl matrinic butanes as potential anti-coxsackievirus agents. Eur. J. Med. Chem. 2017;126:133–142. doi: 10.1016/j.ejmech.2016.09.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.