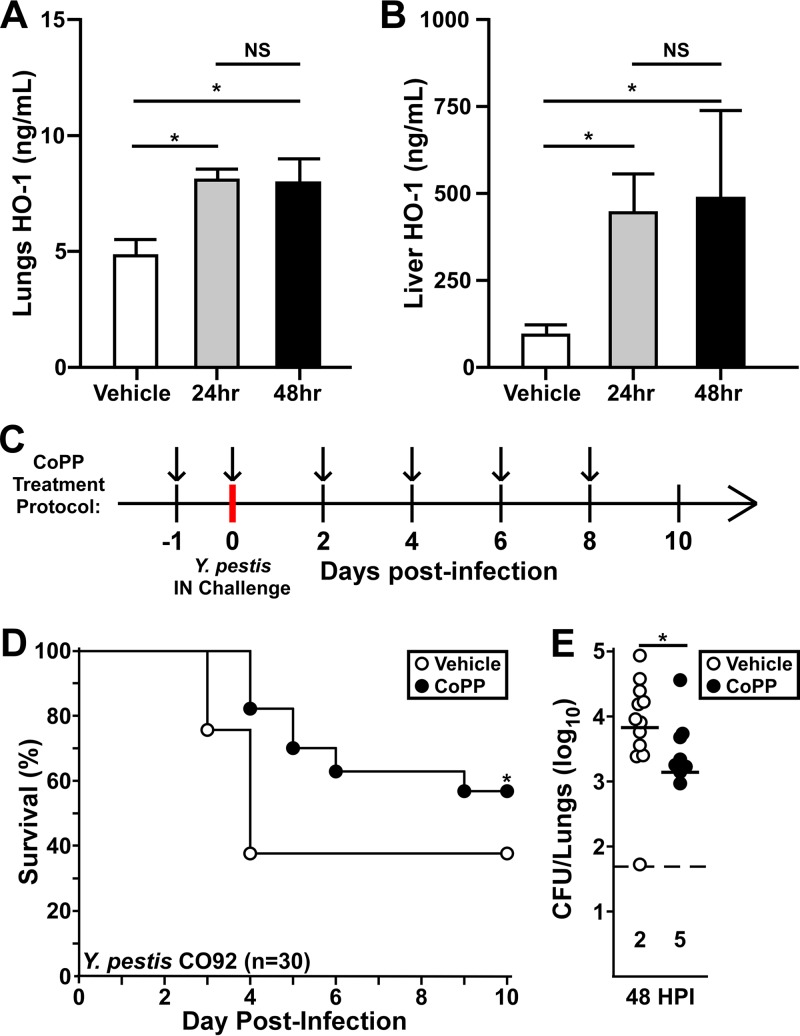
FIG 1.
CoPP is protective against pneumonic plague in mice. (A and B) Groups of five mice (male and female) were provided CoPP treatment by intraperitoneal injection. Comparison between daily (24 h) and every-other-day (48 h) dosing was assessed by measuring HO-1 in the lung (A) and liver (B) homogenates 24 h after the second treatment. The data shown were collected in two independent trials (n = 10 per group). The data represent means with error bars showing standard deviations; statistical significance was evaluated by one-way ANOVA, followed by Dunnett’s multiple-comparison test (*, P < 0.05). (C) Dosing protocol for the mouse studies: i.p. treatment with CoPP (5 mg/kg, black arrows) or PBS vehicle was provided 24 h prior to challenge and on the day of challenge. Additional treatments were provided every other day for the duration of the 10-day observation period. (D and E) Groups of 10 C57BL/6 mice were treated with vehicle or CoPP following the protocol used for panel C and then challenged by intranasal infection with 5,000 to 8,000 CFU Y. pestis CO92. (D) Mice were monitored for survival for 10 days. The data shown were combined from three independent trials (n = 30 per group); the data were evaluated by a Gehan-Breslow log rank test (*, P < 0.05). (E) Groups of three to five male and female C57BL/6 mice, challenged and treated as shown for panel C, were euthanized at 48 h postinfection (hpi), and the bacterial titer was determined by serial dilution and plating. The dashed line represents the limit of detection, and the numbers below the line indicate the numbers of mice with undetectable bacteria. The data shown were collected in three independent trials (n = 13 per group); bars indicate the median. The data were evaluated by the Mann-Whitney test (*, P < 0.05).