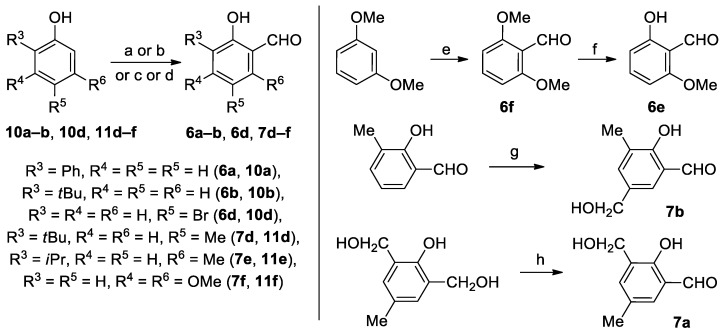
Scheme 3.
The key aldehydes 6a–b, 6d–f, 7a–b, 7d–f preparation. Reagents and conditions: a) (i) NaH, ClMOM, THF, 0 °C; (ii) LICTMEDA, DEE, 0 °C, 1h; (iii) DMF, −75 °C; (iv) HCl, RT, 1h (carried out in accordance with [54]) b) (i) iPrNCO, cat. DMAP, THF, reflux; (ii) TMEDATMSOTf, DEE, RT; (iii) LICTMEDA, −78 °C, 1h; (iv) DMF, −78 °C; (v) NaOH, EtOH, RT; (vi) HCl, −78 °C to RT, total 86% (carried out in accordance with [62]), c) (i) KOH or NaOH, CHCl3, EtOH and/or H2O, 60–65 °C; (ii) HCl (carried out in accordance with [55,64]), d) (i) paraformaldehyde, toluene, cat., SnCl4 and (n-Bu)3N, RT to 100 °C, 8h at 100 °C; (ii) HCl aq. (carried out in accordance with [60]), e) (i) LICTMEDA, THF, 0 °C; (ii) DMF, 0 °C; (iii) HCl (carried out in accordance with [58]), f) AlCl3, DCM (carried out in accordance with [57]), g) HCHO, H2O, 80 °C, 1h (carried out in accordance with [56]), h) MnO2, acetone, RT, 6h (carried out in accordance with [59]).