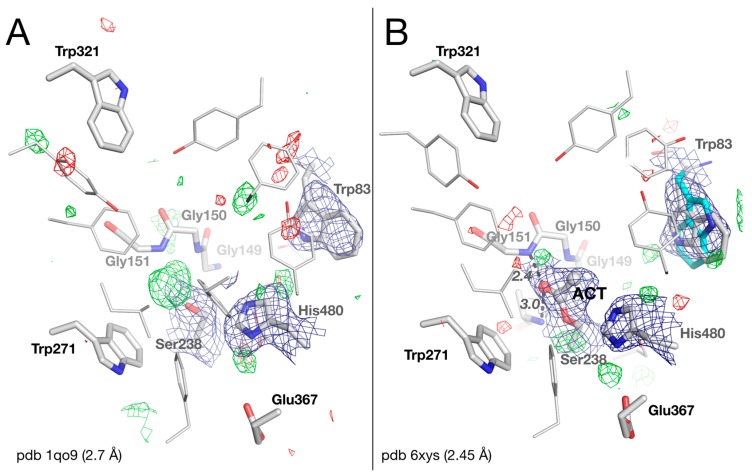
Figure 2.
Active-site gorge in the original (A) and updated (B) structures of native DmAChE. Residues of the catalytic triad (Glu367/His480/Ser238), of the oxyanion hole (Gly150/Gly151/Ala239), and key residues of the peripheral site (Trp321), acyl-binding pocket (Trp271), and choline-binding pocket (Trp83), are represented as sticks, with carbons in white, nitrogens in blue, and oxygens in red. The alternative conformation of Trp83 is depicted with carbons in cyan. The acetyl (ACT) is represented as balls and sticks. H-bonds are depicted as black dashes, with distances in Å. The meshes represent the 2 |Fo| – |Fc| map (1 σ blue) and the |Fo| – |Fc| difference map (3 σ green /−3 σ red).