Abstract
The aim of the present study is to investigate changes of interferon (IFN) production occurring over the first 48 h after infection of peripheral blood mononuclear cells (PBMCs) with severe acute respiratory syndrome (SARS) coronavirus (CoV) and to compare these changes to those induced by well-established IFN-inducing viruses, such as vesicular stomatitis (VSV) and Newcastle viruses (NDV). Experiments have been carried out using PBMCs of 10 different healthy donors. The results showed that the antiviral activity of IFN contained in the supernatant of SARS-CoV-infected PBMCs was lower than those induced by VSV and NDV. Consequently, SARS-CoV induces a lower synthesis of IFN-α, -β and -γ compared to VSV and NDV. Characterization of the profile of IFN-α subtypes genes expression in SARS-CoV-infected PBMCs demonstrated that the level of IFN-α2 and −6 subtypes were higher compared to other IFN-α subtypes namely, IFN-α5, −8, −10, −13/1, −17, and −21. In conclusion, SARS-CoV induces IFNs to a less extent compared to VSV and NDV, thus suggesting that the IFN system does play a limited role in early host defense against SARS-CoV infection.
Key Words: Interferon, SARS-CoV, Interferon-β, Interferon-α, Interferon-γ, Interferon-α subtypes
Severe acute respiratory syndrome (SARS) is a newly identified infectious disease and its causative agent has been convincingly identified as a new member of the Coronaviridae family called SARS-CoV [1]. One fundamental aspect of the innate immune response against viruses is the secretion of type I interferons (IFNs), which induce cellular mechanisms of resistance against viruses and activate host immune responses [2].
The IFNs are a large family of multifunctional cytokines involved in the antiviral response, regulation of cell growth and activation of the immune system [2]. IFN is induced after virus infection, especially by RNA viruses [2], including the members of the Coronaviridae family such as 229E [3].
The role of the immune response in general and specifically of IFN in SARS-CoV pathogenesis has been poorly investigated so far. Since many viruses evolved specific mechanisms for countering the IFN response [4], the efficiency with which viruses counter the cellular antiviral defense mechanisms is an important contributory factor to viral infection pathogenesis. Thus, it is important to investigate the SARS-CoV interactions that lead to infection and especially the mechanism by which the host is capable of clearing out the infection.
In light of the above considerations, we planned to investigate the production of IFN type I and II in response to SARS-CoV (HSR-1 strain) infection of peripheral blood mononuclear cell (PBMC) cultures derived from healthy controls. The strain HSR-1 of SARS-CoV, used in this study, was obtained by inoculating Vero cells with a sputum specimen from an Italian patient affected by a severe form of pneumonia of unknown etiology, who had a history of travel from Vietnam to Italy in March 2003 [5]. PBMCs were chosen to perform the experiments because several studies indicated that SARS-CoV is able to infect in vitro PBMCs and to determine virus-specific RNA production [6, 7, 8]. In order to make a comparison, we performed control experiments in which the same cells were induced to produce IFN by vesicular stomatitis (VSV) and Newcastle disease (NDV) viruses. We used these viruses as control because of their high and well-known capacity of IFN induction.
Briefly, human PBMCs were isolated from buffy coats of 10 blood donors provided by the blood bank of the ‘Sapienza’ University of Rome. Blood was collected in EDTA tubes, and PBMCs were separated by Ficoll-Hypaque gradient sedimentation. PBMCs (5 × 106) were plated in 6-well plates (Falcon 353046 Multiwell 6-Well; Becton-Dickinson, Franklin Lakes, N.J., USA) and infected with 100 μl of SARS-CoV at a MOI of 0.1 in serum-free medium MEM (Sial, Italy) supplemented with 2 mm l-glutamine (Sial, Italy) and 50 μg/ml of gentamycin (Sial, Italy). After adsorption at 37° for 1 h, the excess virus inoculum was removed, the cells were washed with phosphate-buffered saline, and the wells topped up with medium plus 2% fetal calf serum (Sial, Italy) to 0.1 ml/well. Cells were cultured for 24 and 48 h after infection with SARS-CoV. As stated before, control experiments with VSV and NDV at the same MOI were carried out. For safety precautions, all culture supernatants, collected 24 and 48 h post-infection (p.i.), were UV irradiated for 1 h to inactivate residual infectious virus before IFN induction analysis. Cells were collected 24 and 48 h after virus infection and were frozen at −80° for IFN-α subtype mRNA expression analysis (see below).
IFN activity was determined at 24 and 48 h p.i. evaluating the reduction of cytopathic effect on bovine Madin-Darby bovine kidney cells by VSV. In brief, 3 × 104 cells were seeded into each well of 96-well plates (Falcon 353072 Microtest 96, Becton Dickinson) and incubated with threefold serial dilution of supernatant samples for 18 h at 37°. Following incubation, cells were challenged with VSV at a MOI of 0.5 and the plates were incubated at 37° for 18 h. Virus-induced cytopathic effects were assessed by microscopic examination. The monolayer's cell was then stained with crystal violet in 20% ethanol. The dye taken up by the cells was eluted with 33% acetic acid and its absorbance measured at 540 nm with an ELISA microplate reader. IFN concentrations were expressed as inverse dilution that provided 50% of cell protection against viral-induced cytopathic effect.
figure 1a shows the IFN-inducing capacity of SARS-CoV (HSR-1 strain). It can be seen that VSV and NDV induce a higher yield of IFN activity at both 24 and 48 h after infection of PBMCs derived from healthy donors than SARS-CoV (p < 0.05 by Student's t test).
Fig. 1.
Induction of IFN after infection of PBMCs derived from 10 healthy donors with SARS coronavirus (CoV, HSR-1 strain) at a MOI of 0.1. The same experiments were conducted with VSV and NDV viruses. After 24 and 48 h p.i., supernatants were collected and IFNs were detected by biological assay (a) and different ELISA tests (b-d). The values expressed are mean of values obtained in each culture of PBMCs. * p < 0.05 compared with VSV and NDV using Student's t test. ** Not significantly different with NDV at 24 h p.i.
Next, we characterized the antiviral activity in the supernatant of PBMC cultures stimulated with SARS-CoV by analyzing the IFN-α, -β and -γ production using different ELISA kits (Human IFN-α, -γ ELISA Kits, PBL Biomedical Laboratories, Tema Ricerca, Italy). Assays were carried out as recommended by the manufacturer's instructions. The results show that SARS-CoV elicits a significantly lower production of IFN-α and -β compared to VSV and NDV (p < 0.05 by Student's t test; fig. 1b, c). The only exception being the amount of IFN-β induced at 24 h by SARS-CoV which is lower, but not significantly, than that induced by NDV.
IFN-γ levels in supernatant cultures from PBMCs infected with SARS-CoV show no significant differences compared to those induced by VSV or NDV (fig. 1d). However, there was again a trend towards lower production of IFN-γ in PBMCs infected with SARS-CoV, compared to VSV and NDV. In addition, we observed higher levels of IFN-β in supernatants of PBMCs infected with SARS-CoV at 24 h than at 48 h whereas IFN-α and -γ levels remained rather stable up to day 2 p.i.
Next we investigated the transcriptional induction of the main IFN-α subtypes in PBMCs infected with SARS-CoV by real-time reverse transcript-polymerase chain reaction (RT-PCR Taqman) using the ABI 7000 sequence detector (Applied Biosystems, Monza, Italy). Briefly, total cellular RNA was extracted from 5 × 106 cells using phenol and guanidine isothiocyanate reagent (TRIzol, Gibco BRL, Grand Island, N.Y., USA), following the manufacturer's instructions. RNA was then reverse transcribed using a high-capacity cDNA archive kit (Applied Biosystems). Next, we added the following primer pair and probes for IFN-α subtypes [9] to the universal PCR master mix (Applied Biosystems) at 300 and 100 nm, respectively, in a final volume of 50 μl.
The co-amplification of the β-glucuronidase gene (Assay-On-Demand, Hs99999908_m1, Applied Biosystems) was used to control the total amount of RNA extracted from PBMCs. The relative amount of each transcript, normalized to β-glucuronidase mRNA, was calculated by using the arithmetic formula 2−δCt or 2−δδCt according to the supplier's guidelines (Applied Biosystems).
PBMCs isolated from healthy donors were found to express a low quantity of IFN-α subtypes. This constitutive expression is variable depending on the examined IFN-α subtype. Specifically the IFN-α subtype values in unstimulated PBMCs, determined using the equation 2−δCt according to the supplier's guidelines, range between 0.25 and 1.56 for the healthy subjects.
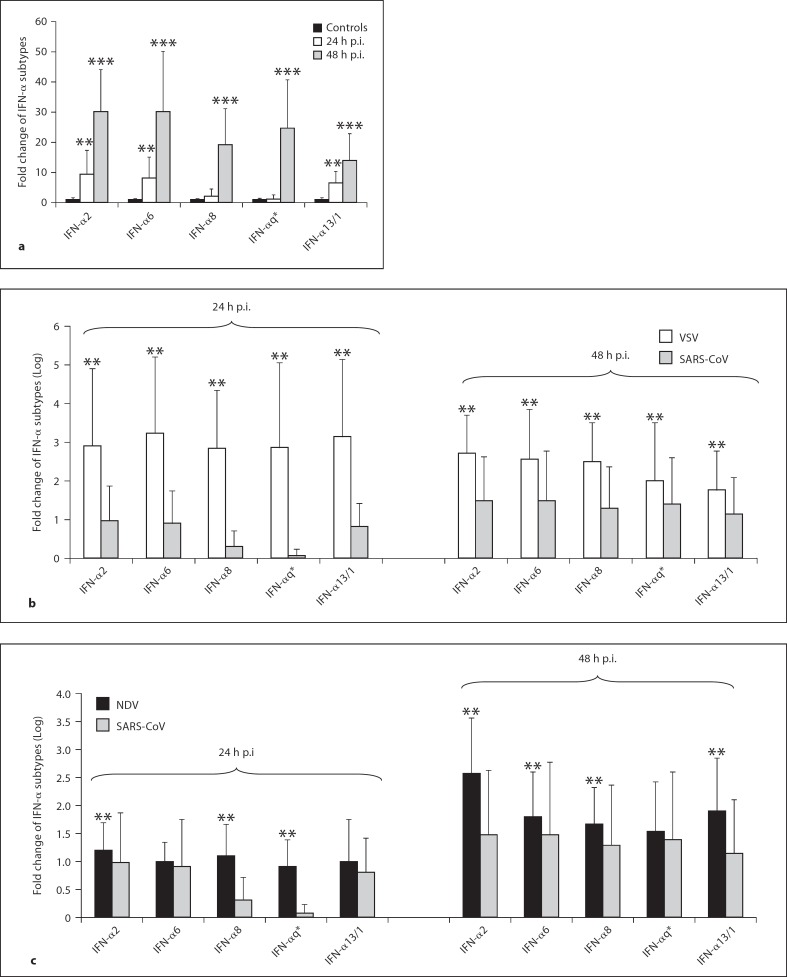
The results also show, for the first time to our knowledge, that SARS-CoV in vitro infection of PBMCs leads to the transcriptional induction of several IFN-α subtypes and that they are induced to a different extent (fig. 2a). In particular, among the subtypes detected at 24 h p.i., IFN-α2 and −6 are the major transcripts followed by IFN-α13/1 and finally IFN-α5, −8, −10, −17, and −21 as minor transcribed subtypes. The difference between the level of expression of IFN-α subtypes 2, 6 and 13/1 measured at 24 h after infection with SARS-CoV is statistically significant (p < 0.05 by Student's t test). All the IFN-α subtypes reached much higher levels at 48 h p.i. than those measured in controls and at 24 h p.i. (p < 0.05 by Student's t test).
Fig. 2.
Expression of IFN-α subtype mRNAs in PBMCs from 10 healthy donors after SARS coronavirus (CoV, HSR1 strain) infection at a multiplicity of infection of 0.1 (a). The same experiments were conducted with VSV and NDV viruses (b, c). At both 24 and 48 h after infection, cells were harvested and RNA was isolated using TRIzol reagent. TaqMan reverse transcriptase PCR was used to quantify IFN-α subtype mRNAs. The data, normalized to β-glucuronidase mRNA, were calculated by using the arithmetic formula 2−δδCt according to the supplier's guidelines. * IFN q = IFN-α5, −10, −17, and −21. a ** p < 0.05 compared with expression of IFN-α subtypes in uninfected PBMCs (controls) using Student's t test. *** p < 0.05 compared with expression of IFN-α subtypes in uninfected PBMCs (controls) and at 24 h p.i. using Student's t test. b, c ** p < 0.05 compared with expression of IFN-α subtypes in PBMCs infected with SARS-CoV using Student's t test.
As expected, a significant production of IFN-α subtypes in PBMCs infected with VSV or NDV at 24 h as well as at 48 h was observed (p < 0.05 by Student's t test; fig. 2b, c). Interestingly, we found that, over a time course of 24 and 48 h of infection, lower levels of all of the IFN-α subtypes investigated were transcribed in PBMCs infected with SARS-CoV when compared with those induced by VSV and NDV. The ratio values, expressed as Log values, in the IFN-α subtype levels between VSV and SARS-CoV after PBMCs infection were variable. The mean value at 24 h was 2.38 (range 1.93-2.78) and at 48 h it was 0.94 (range 0.6-1.2). The same analysis performed with NDV revealed that mean value at 24 and 48 h were 0.42 and 0.53, respectively.
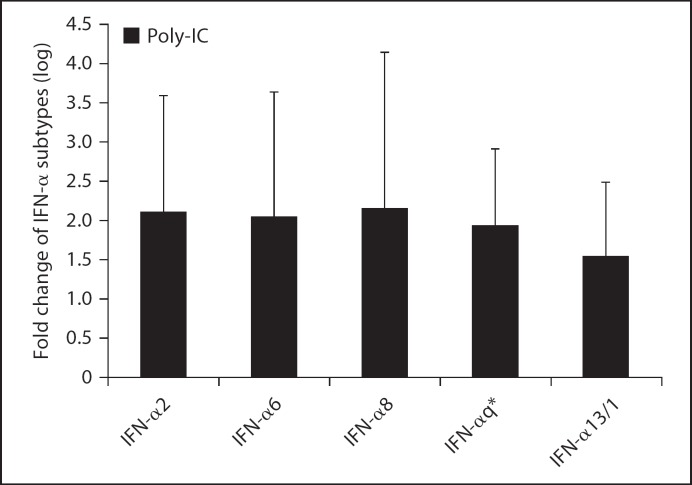
Such findings, which are consistent with previous observations [9], support the idea that the expression level of each IFN-α subtype is dependent on the kind of viral stimulus. To further support the view that SARS-CoV is a poor inducer of IFN-α subtypes and to gain new insights into the phenomenon, the increase of all IFN-α subtypes was measured in PBMCs collected from 5 healthy donors after transfection with double-stranded RNA synthetic analogue such as polyriboinosinic-polyribocytidylic acid (poly-IC) for 24 h. The results of these experiments are shown in figure 3 where it can be seen that relevant amounts of different IFN-α subtypes can be measured in cells transfected as above. This observation indirectly underlines that SARS-CoV infection of PBMCs was accompanied by a low induction of IFN-α mRNA subtypes and suggests that toll-like receptor 3 (TLR3) together with other pattern recognition receptors could be involved in the signaling leading to expression of type I IFN in PBMCs.
Fig. 3.
Gene expression of IFN-α subtypes in PBMCs from 5 healthy donors after stimulation with 50 μg/ml of poly-IC and DEAE-dextran (50 μg/ml). At 24 h, cells were harvested and RNA was isolated using TRIzol reagent. TaqMan reverse transcriptase PCR was used to quantify IFN-α subtypes mRNAs. The data, normalized to β-glucuronidase mRNA, were calculated by using the arithmetic formula 2−δδCt according to the supplier's guidelines. * IFN q = IFN-α5, −10, −17, and −21.
Altogether, the data presented in this study, along with previous results [10, 11, 12, 13, 14, 15, 16, 17, 18, 19], demonstrate that SARS-CoV is a weak inducer of IFN-α, -β and -γ. The potential limitations of our study include the fact that we did not compare IFN response in SARS-CoV-infected PBMCs with those in other relevant human virus. In line with this, it has been reported that in contrast to human coronavirus 229E and influenza A virus (H1N1) there was little or no induction of IFN-β in SARS-CoV-infected macrophages [20]. Moreover, it has been shown that SARS-CoV infection of primary human macrophages is associated with a strong induction of chemokines without an associate type I IFN response compared to H5N1 infection [21]. This finding is consistent with the observation that SARS-CoV proteins that prevent IFN induction do exist [22, 23, 24]. In particular, the expression of nsp1 protein of SARS-CoV prevents Sendai virus-induced endogenous IFN-β mRNA accumulation without inhibiting dimerization of IFN regulatory factor 3, a protein that is essential for the activation of the IFN-β promoter [22]. Furthermore, nsp1 expression promotes degradation of RNA transcripts and host endogenous mRNAs, leading to a stronger host protein synthesis inhibition. In addition, Kopecky-Bromberg et al. [24] found that SARS-CoV open reading frame (ORF) 3b, ORF 6, and N proteins inhibit the expression of IRF-3, suggesting that, like other highly pathogenic viruses, SARS-CoV genome encodes more than one protein able to inhibit IFN.
Therefore, altogether these results may help to explain the rapid rise in virus titers during the initial phase of SARS disease. In agreement with the above consideration, it has been reported that several IFN-stimulated genes, such as PKR, GBP-1/2, CXL-10/11, and JAK/STAT signal pathway were down-regulated in SARS patients with acute severe phase compared to patients with the convalescent phase [11].
However, in contrast with our results and many other results obtained with in vitro experiments [10, 15, 16, 17, 18, 19], it has been recently demonstrated that SARS-CoV induced a wide range of type I IFNs and nuclear translocation of phosphorylated signal transducer and activator of transcription 1 in the lungs of macaques [25]. Moreover, it has also been reported by Okabayashi et al. [26] that the IFN system was not suppressed by SARS-CoV infection. The reasons for these discrepancies are still unclear. Several factors should be taken into account. These include differences in: (i) cells types used to investigate SARS-CoV capability to induce IFN; (ii) methods employed to determine IFN induction; (iii) timing of sample collections for the analysis of IFN induction with respect to SARS-CoV infections, and (iv) type of virus used to make a comparison with IFN production in SARS-CoV infection. Further studies are required to gain a better understanding of the activation of IFN system during SARS-CoV infection.
Importantly, the results of this study also demonstrate that lower levels of all of the IFN-α subtypes investigated were transcribed in PBMCs infected with SARS-CoV, in contrast with those induced by VSV and NDV. In addition, we observed that SARS-CoV induce a characteristic profile of IFN-α subtype production.
It is generally believed that the transcription of different IFN-α subtypes is modulated through multiple signaling pathways depending on the virus and the type of cells infected. However, the expression and function of IFN-α subtypes during infection with highly pathogenic viruses have been poorly investigated. Interestingly, hepatitis C virus (HCV) infection has been reported to be associated with low IFN-α5 mRNA levels, which is the main IFN-α subtype expressed in the liver [27]. Our results show that IFN-α5 mRNA production at 24 h p.i. was lower compared to the other IFN-α subtypes investigated, when induced with SARS-CoV too. On the contrary, the levels of IFN-α6 and −2 are higher, although it has been reported that IFN-α6 is up to 52 times less activated than IFN-α2 after infections of PBMCs with either herpes simplex virus or other different viruses of the Paramixoviridae family [9].
It is well known that different IFN-α subtypes possess distinct antiviral and antiproliferative effects both in vitro and in vivo [28, 29, 30, 31, 32]. It has been reported that IFN-α8 has the most potent antiviral activity against murine encephalomyelitis virus in various human cell lines, while IFN-α1 is the least efficient [33]. In agreement, Koyama et al. [34] demonstrated that IFN-α8 was the most effective of the IFN-α subfamily against intracellular hepatitis C virus replication.
In light of the above findings, the fact that SARS-CoV induced lower levels of IFN-α8 compared to IFN-α2, −6 and −13/1 could suggest a scenario in which during SARS the virus affect selectively the expression of specific IFN-α subtypes with the most biological activity. The impaired expression of specific IFN-α subtypes caused by SARS-CoV could contribute to the main immunopathological processes involved in SARS. A better understanding of SARS-CoV strategies to alter IFN-α subtype expression may elucidate the mechanism underlying virus-induced airway disease.
In summary, our data, together with previous results, demonstrate that SARS-CoV (HSR-1 strain) induces a weak IFN response in comparison with well-established IFN-inducing viruses, such as those for VSV and NDV. This phenomenon has also been described for murine hepatitis coronavirus, which is closely related to SARS-CoV [14]. Interestingly, our results further indicate that SARS-CoV-induced activation of the IFN system in human PBMCs is associated with a selective expression of individual IFN-α subtypes, IFN-α2 and −6 being the most abundant subtypes detected at 24 h p.i. Our results will hopefully make a contribution to further studies of SARS pathogenesis.
Acknowledgement
This work was supported in part by a grant to G.A. from University ‘Sapienza’ (Fondi Progetti di ricerca universitaria quota 60%).
References
- 1.Satija N, Lal SK. The molecular biology of SARS coronavirus. Ann NY Acad Sci. 2007;1102:26–38. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chelbi-Alix MK, Wietzerbin J. Interferon, a growing cytokine family: 50 years of interferon research. Biochimie. 2007;89:713–718. doi: 10.1016/j.biochi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pitkäranta A, Hovi T. Induction of interferon in human leukocyte cultures by natural pathogenic respiratory viruses. J Interferon Res. 1993;13:423–426. doi: 10.1089/jir.1993.13.423. [DOI] [PubMed] [Google Scholar]
- 4.Weber F, Haller O. Viral suppression of the interferon system. Biochimie. 2007;89:836–842. doi: 10.1016/j.biochi.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicenzi E, Canducci F, Pinna D, Mancini N, Carletti S, Lazzarin A, Bordignon C, Poli G, Clementi M. Coronaviridae and SARS-associated coronavirus strain HSR-1. Emerging Infect Dis. 2004;10:413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castilletti C, Bordi L, Lalle E, Rozera G, Poccia F, Agrati C, Abbate I, Capobianchi MR. Coordinate induction of IFN-α and -γ by SARS-CoV also in the absence of virus replication. Virology. 2005;341:163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilla M, Harcourt BH, Hickman CJ, McGrew M, Tamin A, Goldsmith CS, Bellini WJ, Anderson LJ. SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 2005;107:93–101. doi: 10.1016/j.virusres.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng LF, Hibberd ML, Ooi EE, Tang KF, Neo SY, Tan J, Murthy KR, Vega VB, Chia JM, Liu ET, Ren EC. A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis. 2004;4:34. doi: 10.1186/1471-2334-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loseke S, Grage-Griebenow E, Wagner A, Gehlhar K, Bufe A. Differential expression of IFN-α subtypes in human PBMCS: evaluation of novel real-time PCR assays. J Immunol Methods. 2003;276:207–222. doi: 10.1016/s0022-1759(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 10.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, Peiris JS. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu SY, Hu YW, Liu XY, Xiong W, Zhou ZT, Yuan ZH. Gene expression profiles in peripheral blood mononuclear cells of SARS patients. World J Gastroenterol. 2005;11:5037–5043. doi: 10.3748/wjg.v11.i32.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome. Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versteeg GA, Bredenbeek PJ, van den Worm SH, Spaan WJ. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology. 2007;361:18–26. doi: 10.1016/j.virol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang BS, Chan KH, Cheng VC, Woo PC, Lau SK, Lam CC, Chan TL, Wu AK, Hung IF, Leung SY, Yuen KY. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J Virol. 2005;79:6180–6193. doi: 10.1128/JVI.79.10.6180-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler T, Matikainen S, Rönkkö E, Osterlund P, Sillanpää M, Sirénl J, Fagerlund R, Immonen M, Melén K, Julkunen I. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J Virol. 2005;79:13800–13805. doi: 10.1128/JVI.79.21.13800-13805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. Gen Virol. 2006;87:1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel M, Weber F. Inhibition of cytokine gene expression and induction of chemokine genes in non-lymphatic cells infected with SARS coronavirus. J Virol. 2006;293:17. doi: 10.1186/1743-422X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, Peiris JS. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris M. Pathogenesis of avian flu H5N1 and SARS. Novartis Found Symp. 2006;279:56–60. [PubMed] [Google Scholar]
- 22.Spiegel M, Pichlmair A, Martínez-Sobrido L, Cros J, García-Sastre A, Haller O, Weber F. Inhibition of β-interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J Virol. 2005;79:2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamitani W, Narayanan K, Huang C, Lokugamage K, Ikegami T, Ito N, Kubo H, Makino S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc Natl Acad Sci USA. 2006;103:12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopecky-Bromberg SA, Martinez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Lang A, Baas T, Teal T, Leijten LM, Rain B, Osterhaus AD, Haagmans BL, Katze MG. Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathog. 2007;3:112. doi: 10.1371/journal.ppat.0030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabayashi T, Kariwa H, Yokota S, Iki S, Indoh T, Yokosawa N, Takashima I, Tsutsumi H, Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrea E, Alberdi A, Castelruiz Y, Boya P, Civeira MP, Prieto J. Expression of interferon-α subtypes in peripheral mononuclear cells from patients with chronic hepatitis C: a role for interferon-α5. J Viral Hepat. 2001;8:103–110. doi: 10.1046/j.1365-2893.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 28.Evinger M, Rubinstein M, Pestka S. Antiproliferative and antiviral activities of human leukocyte interferons. Arch Biochem Biophys. 1981;210:319–329. doi: 10.1016/0003-9861(81)90195-8. [DOI] [PubMed] [Google Scholar]
- 29.Fish EN, Banerjee K, Stebbing N. Human leukocyte interferon subtypes have different antiproliferative and antiviral activities on human cells. Biochem Biophys Res Commun. 1983;112:537–546. doi: 10.1016/0006-291x(83)91498-5. [DOI] [PubMed] [Google Scholar]
- 30.Foster GR, Rodrigues O, Ghouze F, Schulte-Frohlinde E, Testa D, Liao M J, Stark GR, Leadbeater L, Thomas HC. Different relative activities of human cell-derived interferon-alpha subtypes: IFN-α8 has very high antiviral potency. J Interferon Cytokine Res. 1996;16:1027–1033. doi: 10.1089/jir.1996.16.1027. [DOI] [PubMed] [Google Scholar]
- 31.Nyman TA, Tolo H, Parkkinen J, Kalkkinen N. Identification of nine interferon-α subtypes produced by Sendai virus-induced human peripheral blood leucocytes. Biochem J. 1998;329:295–302. doi: 10.1042/bj3290295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schanen C, Chieux V, Lobert PE, Harvey J, Hober D. Correlation between the antivirus-induced cytopathic effect activity of interferon-α subtypes and induction of MxA protein in vitro. Microbiol Immunol. 2006;50:19–24. doi: 10.1111/j.1348-0421.2006.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto S, Yano H, Sanou O, Ikegami H, Kurimoto M, Kojiro M. Different antiviral activities of IFN-α subtypes in human liver cell lines: synergism between IFN-α2 and IFN-α8. Hepatol Res. 2002;24:99. doi: 10.1016/s1386-6346(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 34.Koyama T, Sakamoto N, Tanabe Y, Nakagawa M, Itsui Y, Takeda Y, Kakinuma S, Sekine Y, Maekawa S, Yanai Y, Kurimoto M, Watanabe M. Divergent activities of interferon-α subtypes against intracellular hepatitis C virus replication. Hepatol Res. 2006;34:41–49. doi: 10.1016/j.hepres.2005.10.005. [DOI] [PubMed] [Google Scholar]