Abstract
Mouse hepatitis virus (MHV) A59 infection which causes acute encephalitis, hepatitis, and chronic demyelination, is one of the experimental models for multiple sclerosis. Previous studies showed that lethal infection of β2-microglobulin ‘knockout’ (β2M(-/-)) mice required 500-fold less virus and viral clearance was delayed as compared to infection of immunocompetent C57Bl/6 (B6) mice. To investigate the mechanism of the increased susceptibility of β2M(-/-) mice to MHV-A59, we studied organ pathology and the distribution of viral antigen and RNA during acute and chronic infection. A59-infected β2M(-/-) mice were more susceptible to acute encephalitis and hepatitis, but did not have increased susceptibility to demyelination. Viral antigen and RNA distribution in the brain was increased in microglia, lymphocytes, and small vessel endothelial cells while the distribution in neurons and glia was similar in β2M(-/-) mice and B6 mice. Acute hepatitis and thymus cortical hypoplasia in β2M(-/-) mice were delayed in onset but pathologic changes in these organs were similar to those in B6 mice. The low rate of demyelination in β2M(-/-) mice was consistent with the low dose of the virus given. A less neurotropic virus MHV-2, caused increased parenchymal inflammation in β2M(-/-) mice, but without demyelination. Thus, CD8+ cells were important for viral clearance from endothelial cells, microglia and inflammatory cells, but not from neuronal and glial cells. In addition, CD8+ cells played a role in preventing the spread of encephalitis.
Key Words: Coronaviruses, Nidoviruses, Immunohistochemistry, In situ hybridization, Pathogenesis
Introduction
Coronavirus mouse hepatitis virus (MHV) is a single-stranded enveloped RNA virus of positive polarity [1, 2, 3]. MHV infection of mice provides an excellent model for studying acute and chronic persistent diseases of the central nervous system (CNS) [2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Certain strains of the MHV (such as A59 and JHM), produce a chronic demyelinating disease, probably due to immune mediated mechanisms, sharing many of the pathologic hallmarks with those of the human demyelinating disease multiple sclerosis (MS) [12, 13, 14].
The nature of the immune component responsible for the demyelinating process in MHV is not clearly understood. MHV infection of the CNS induces enhanced expression of MHC class I antigens on brain cells in vitro and in vivo [15, 16, 17, 18, 19]. However, the significance of MHC induction in mHV infection is still not clear. It can potentially enable cytotoxic phenomena on neural cells including oligodendrocytes, by CD8+ cells which require recognition by MHC class I antigens on the surface of the target cells. In SCID mice, in which the B- and T-cell immune components are nonfunctional, infection by MHV does not produce significant demyelination [20], suggesting that a subset of the affected immune cells in SCID mice are important for demyelination. The cells which affect the rate and quality of the demyelinating process were characterized as Thy1+ cells [21], presumably a subset of T cells.
Cytotoxic, CD8+, T cells, were shown to be important for clearance of MHV infection [22], although, perforin-mediated cytolysis was not essential for viral clearance [23]. In order to examine whether CD8+ cells also affected demyelination, we previously studied the ability of MHV-A59 to produce demyelination in β2-microglobulin ‘knockout’ (β2M(-/-)) mice [24, 25]. Since the β2-microglobulin is part of the MHC class I protein, disruption of β2-microglobulin expression interferes with normal expression of MHC class I and the development of functional CD8+ cells in β2M(-/-) mice [26]. We found that demyelination developed in some β2M(-/-) mice following infection with a lethal dose of MHV-A59, thus CD8+ cells were not an absolute requirement for demyelination [24]. However, the pathogenesis of the disease and the reasons for the increased susceptibility of β2M(/-) mice to MHV infection were still unknown. Thus, in the present study we investigated the effect of lack of CD8+ cells on organ pathology, and the cellular reservoirs for viral infection, in both acute and chronic MHV infection of β2M(-/-) mice.
Materials and Methods
Viruses Cells and Mice
MHV-A59 [10] and MHV-2 [27] were used in this study. Viruses were propagated and assayed on L2 murine fibroblast cells at 37 °C, propagated in DMEM (Gibco) containing 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin.
Four-week-old, C57Bl/6 (B6) mice (Jackson Laboratories, Bar Harbor, Me., USA), were used. β2M(-/-) mice were originally obtained from Dr. Beverly Koller [26], and were inbred at the University of Pennsylvania. Mice were produced by the combination of B6 and 129/J [24]. Additional β2M(-/-) mice were purchased from Jackson Laboratories. The lethal dose of MHV-A59 that killed 50% of the mice (LD50 dose) was found to be 5 PFU/mouse, compared to 3,000 PFU in B6 mice [24]. Groups of 3-5 per time point, 4-week-old mice, were inoculated intracerebrally (IC) with 20 μ of the viruses, and were sacrificed by methoxyflurane overdose at various intervals postinoculation (1, 3, 5, 7, 9, 11, 15, 30 days postinoculation) and perfused intracardially with PBS and 10% phosphate-buffered formalin. Control mice were mock-infected with the same volume of sterile L2 cell lysate not containing virus. To ensure that the colony of β2M(-/-) mice was truly deficient of CD8+ cells, random analyses of cells from the spleen and lymph nodes from uninfected β2M(-/-) mice were carried out. These populations of cells were consistently negative for the presence of CD8+ or β2-microglobulin as assayed by fluorescence-activated cell-sorting assays [24].
Histopathology
Mice were perfused with 10 ml PBS, then 10 ml of 10% buffered formalin (Sigma, St. Louis, Mo., USA) and organs, including thymus, liver, brain and spinal cord, were removed and fixed in formalin for at least an additional 48 h. In selected mice the entire block of internal organs was removed and fixed in formalin. Individual organs were then dissected and processed for embedding in paraffin. Tissues were embedded in paraffin, sectioned at 5 μM and stained with hematoxylin and eosin (HE). Brain and spinal cord sections were also stained with Luxol Fast Blue for myelin. For each animal at least 5 coronal sections of brain and multiple sections of cervical, thoracic, and lumbar spinal cord were prepared. Additional mice were perfused with 2% glutaraldehyde (Sigma) and spinal cord sections were embedded in Epon, sectioned at 1 μM and stained with toluidine blue [10]. The sections were examined for evidence of primary demyelination (loss of myelin sheaths surrounding intact neuronal axons).
Viral Titers
For assessment of viral titers, organs were removed from mice aseptically following PBS perfusion and kept frozen at −80 °C. These samples were homogenized, and tested for viral titers by plaque assays in 6-well plates [10, 24].
Immunohistochemistry
Formalin-fixed, paraffin-embedded unstained 5-μM sections were used. Immunohistochemistry was performed using the ABC Elite immunoperoxidase kit (Vector Laboratories, Burlingame, Calif., USA) with diaminobenzidine as substrate and rabbit polyclonal anti-MHV antibodies used at a 1:100 dilution [28]. Control experiments included incubation of sections with the same concentration of an irrelevant antibody of matched haplotype.
Combined in situ Hybridization for MHV RNA and Cell Marker Immunohistochemistry
To detect viral RNA, in situ hybridization was performed on tissue sections from MHV-A59-infected β2M(-/-) mice. The nature of the cell types infected with the virus was determined using a combination of in situ hybridization for viral RNA with cell-specific markers (GFAP antibodies for astrocytes and RCA-1 for microglia and endothelial cells) as previously described [29]. Paraffin-embedded, formalin-fixed tissues were placed on heavy teflon (HTC) slides (Cell-Line Associates, Newfield, N.J., USA) deparaffinized, air-dried and histochemistry performed with anti-GFAP monoclonal antibodies at 1:500 dilution [30], or RCA-1 (Sigma). After permeabilization with pepsin (2 mg/ml in 0.1 N HCl for 15 min at 37 ° C) the slides were washed in 0.1 M Tris buffer (pH 7.4) for 1 min, then in 100% ethanol, and then air-dried. MHV RNA was detected by in situ hybridization with a biotinylated oligonucleotide probe composed of S-gene sequences 5′TATAAGAGTGATTGGCGTCC3′. An oligonucleotide probe derived from the HIV gag gene 5′ATCCTGGGATTAAATAAAATAGTAAGAATGTATAGCCCTAC3′ was used as control. The hybridization mixture contained 1 nmol/100 μl of probe, 5 × SSC, 1% blocking reagent (BMB), 0.1% N-lauroylsarcosine and 0.2% SDS. The slides were covered, incubated on a heat block for 10 min at 95 °C, and transferred to a humidified chamber for 12 h at 45 °C. After incubation the slides were first washed in 2 × SSC, 0.1% SDS for 15 min, and then in 0.1% SDS for 15 min at 37 °C. Slides were then incubated with streptavidin alkaline phosphatase (sheep) for 2h at 37 °C. After incubation, the slides were washed in 0.1 M Tris buffer (pH 7.4). The signal was colorized with NBT/BCIP.
Results
Pathology of Acute Disease in CD8+-Negative Mice Infected with MHV-A59
We previously found that β2M(-/-) mice were significantly more susceptible to infection with MHV-A59, as compared to the two parental strains of mice that were used for the development of these transgenic mice, i.e. B6 and 129/J [24]. Disease in either B6 or 129/J mice was identical. Normal susceptible mice (B6) infected with 5,000 PFU/mouse (1-2 LD50 dose) of the neurotropic virus MHV-A59 developed both encephalitis and hepatitis. Following IC inoculation with 10 PFU of MHV-A59 (2 LD50), β2M(-/-) mice developed clinical signs of acute disease including ruffled fur, hunched position, decreased motility and appetite, lethargy, limb weakness, and eventually death. These clinical signs were similar to the ones observed in B6 mice infected with 5,000 PFU of MHV-A59. Thus, β2M(-/-) mice required 500-fold less virus than B6 mice to produce a similar mortality rate and clinical disease. Previous analysis of viral titers in organs of the two types of mice infected with MHV-A59 showed that β2M(-/-) mice infected with 10 PFU had higher and more sustained titers of virus as compared to B6 mice infected with 5,000 PFU of MHV-A59 [24]. These results were consistent with the hypothesis that CD8+ cells play an important role in MHV clearance from the organs of infected mice.
We next sought to investigate the cellular reservoirs which cause the increased susceptibility to and the increase in virulence of MHV-A59 in β2M(-/-) mice. Therefore, we compared the histopathological changes in β2M(-/-) mice and B6 mice both injected with doses that produce comparable clinical effect, i.e. 1-2 LD50 dose. We also compared the pathological changes in these two strains of mice given the same dose of virus. A dose of 10 PFU of MHV-A59 in B6 mice did not produce any clinical disease and the only pathologic change was minimal meningeal infiltration. A dose of 5,000 PFU of MHV-A59 in β2M(-/-) mice killed all the mice but the pathologic changes obtained at necropsy were similar to the ones seen with 10 PFU.
The histopathologic changes in β2M(-/-) mice infected with 10 PFU of MHV-A59 are summarized in table 1. Between 7 and 13 days postinoculation, 100% of the mice had pathological changes in the brain, liver and thymus. We analyzed all of the other organs for histopathological change in order to determine if organs other than liver and brain may be involved in acute infection in β2M(-/-) mice, providing additional cellular reservoirs for viral replication. No pathologic changes were seen in the heart, lung, gastrointestinal tract, spleen, pancreas, adrenals, genital organs, salivary glands, bone marrow, skin or muscle in any of the mice.
Table 1.
Comparison of pathologic features in β2M(-/-) mice and B6 mice infected with MHV-A59
| Pathology | PID | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 4 | 7-8 | 11 | 13 | 21 | 30 | |
| β2M(-/-) mice | |||||||
| Meningitis | 1/2 | 3/6 | 6/6 | 2/2 | 4/4 | 1/3 | 0/3 |
| Encephalitis | 0/2 | 3/6 | 6/6 | 2/2 | 3/4 | 1/3 | 0/3 |
| Chronic inflammatory demyelination Hepatitis | 0/2 | 0/6 | 5/6 | 2/2 | 3/4 | 2/3 | 14/200 (7%) 0/3 |
| Thymic hypoplasia | 0/2 | 0/2 | 2/5 | 1/1 | 2/2 | 0/2 | 0/3 |
| B6 mice | |||||||
| Meningitis | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Encephalitis | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Chronic inflammatory demyelination Hepatitis | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 64/100 (64%) 0/3 |
| Thymic hypoplasia | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
Each type of mice were infected with approximately 2 LD50 dose of virus (10 PFU for β2M(-/-) mice and 5,000 for B6 mice). The pathologic features are expressed as the number of positive mice per total number of mice examined for this pathologic feature at a particular time point. Demyelination is expressed as the number of demyelinated spinal cord quadrants per total number of spinal cord quadrants.
Examination of the livers of β2M(-/-) mice infected with 10 PFU of MHV-A59 revealed a more prolonged course of acute hepatitis. In B6 mice, hepatitis began 3 days postinfection and was cleared by 2 weeks postinfection; whereas in β2M(-/-) mice, hepatitis was not seen before 7-8 days postinfection. On day 21 postinfection, 2 of 3 mice still exhibited chronic persistent hepatitis, with mononuclear inflammatory infiltrates within foci of damaged hepatocytes and the beginning of microvacuolar lipidization of hepatocytes. Thus acute hepatitis in β2M(-/-) mice was delayed in onset and clearance, but was similar in morphologic aspects and in the extent of tissue damage to acute hepatitis in B6 mice.
Examination of the thymus of β2M(-/-) mice infected with 10 PFU of MHV-A59 showed evidence of cortical depletion of lymphocytes. The thymus cortex was transformed into a skeleton of epithelial cells and blood vessels and only a small number of lymphocytes remaining. The thymic cortex of uninfected or mock-infected β2M(-/-) mice was densely populated with lymphocytes. There were no significant changes in the thymic medulla. These changes were observed between days 7 and 13 postinfection and were similar to the ones observed in B6 mice infected with MHV-A59. Viral titers of thymus from infected mice were negative by plaque assay.
Brain pathology following MHV-A59 infection in B6 mice consisted of focal acute encephalitis, characterized by microglial nodules, neuronophagia and inflammatory infiltrates, mostly lymphocytes, in regions of the brain typically susceptible to MHV-A59 infection. These areas included the olfactory bulbs, the prepiriform and piriform cortex, the septal nuclei, the claustrum, the lateral habenula, the parahippocampal and entorhinal cortex, the subiculum, amygdala, subthalamic nuclei and substantia nigra, the ventral tegmental area, and the locus ceruleus. Areas of the neocortex and cerebellum were generally spared as previously described [10, 28, 31]. Histopathologic examination of the brain of β2M(-/-) mice infected with 10 PFU of MHV-A59 also showed meningoencephalitis with predilection for olfactory-limbic structures similar to the changes seen in MHV-A59-infected B6 mice. However, there was more extensive involvement of the basal ganglia and thalamus of β2M(-/-) mice as compared to B6-infected mice. Both B6 and β2M(-/-) mice showed no significant involvement of neocortex and cerebellum in infected β2M(-/-) mice.
The brain of MHV-A59-infected β2M(-/-) mice was infiltrated by mononuclear inflammatory cells, mostly lymphocytes, as in MHV-A59-infected B6 mice. However, there was more extensive proliferation of rod-shaped microglial cells in involved areas in β2M(-/-) mice as compared to acute encephalitis in B6 mice. Control mock-infected mice did not exhibit any specific pathological changes in any of the organs.
Distribution of Antigen and Viral RNA in Brains of β2M(-/-) Mice Infected with MHV-A59
Since viral titers in brains of β2M(-/-) mice were generally 2 logs higher at the peak of acute infection of the brain [24], we wanted to analyze the spread of viral antigens and genome. We asked whether the number of infected cells was increased and whether additional cell types served as reservoirs for viral replication during infection of β2M(-/-) mice with MHV-A59. We performed immunohistochemistry on brain sections from β2M(-/-) mice, during acute infection with MHV-A59, to detect the presence of viral antigen. Immunohistochemistry in β2M(-/-) mice revealed viral antigen in neurons in a similar intensity and anatomic distribution to that seen in B6 mice [28]. The infected neurons seen during acute encephalitis were generally found in the same anatomic location of the inflammatory infiltrates (as described in the previous section). However, there was increased viral antigen in numerous microglia, lymphocytes, endothelial and meningeal cells in β2M(-/-) mice as compared to B6 mice [28] which rarely presented viral antigens in these cell types during infection with MHV-A59 (fig. 1).
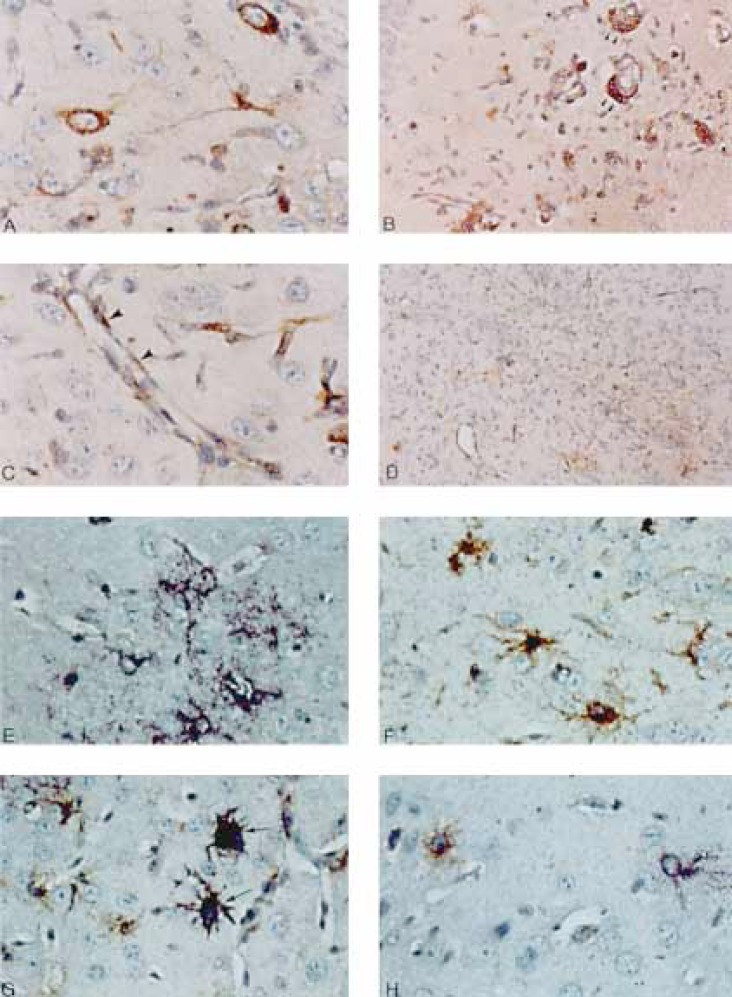
Fig. 1.
A-D Immunohistochemical analysis of MHV-A59-infected β2M(-/-) mouse brains. A Neuronal infection is exhibited by positive immunostaining for MHV antigen in the cytoplasm of parahippocampal neurons. Note the morphological stigmata of neurons: the large cell size, the characteristic oval nucleus with the prominent nucleolus, and the thick axonal and dendritic processes. × 400. B Viral antigen detected in perivascular lymphocytes (arrowheads). × 200. C Viral antigen detected in endothelial cells delineating cerebral small venules and capillaries (arrowheads). × 400. D Area of prominent rod-shaped microglial cell proliferation including many that stain positively for viral antigen. × 100. E-H Combined in situ hybridization for viral genome and GFAP immunohistochemical staining for astrocytes. E GFAP-negative neuronal cells expressing viral genome (purple). Small purple dots represent positive viral genome in cross-sectioned cell processes. × 400. F Uninfected reactive astrocytes which are negative for viral genome by in situ hybridization expressing abundant GFAP by immunohistochemistry. × 400. G Infected astrocytes (arrows) exhibiting both GFAP (by immunohistochemistry with GFAP antibodies) and viral genome (in situ hybridization with MHV-specific probes). × 400. H A neuron expressing viral genome by in situ hybridization (purple) next to a GFAP-positive astrocyte (brown). × 400.
We performed in situ hybridization on tissue sections of MHV-A59-infected β2M(-/-) mice with MHV-specific probes, in order to determine the distribution of viral genome during acute encephalitis. Viral RNA and antigen had similar distribution. The signal of viral RNA in MHV-A59-infected β2M(-/-) mice was seen within neurons in olfactory-limbic areas in addition to many microglia, perivascular lymphocytes, and in capillary endothelial cells. Double staining with GFAP revealed many GFAP-negative cells, positive for viral RNA. There were also many GFAP-positive cells which were negative for viral RNA. Many of these cells had abundant cytoplasm and ramified thick processes characteristic of reactive astrocytes, indicating reactive gliosis. Occasional GFAP-positive cells were also positive for viral RNA suggesting that only a small percent of astrocytes are infected (fig. 1). Thus during acute infection of β2M(-/-) mice with MHV-A59, the distribution of viral RNA and antigen were similar in neurons, microglia endothelial cells and a small number of glial cells including GFAP-positive astrocytes. The pathologic changes during acute meningoencephalitis also included reactive gliosis. In situ hybridization for viral genome combined with RCA-1 staining showed viral genome in microglia and endothelial cells, similar to the immunohistochemical results for viral antigens.
Liver sections from MHV-A59-infected β2M(-/-) mice were also examined by immunohistochemistry for the presence of viral antigen. Staining revealed similar distribution of viral antigen in β2M(-/-) and B6-infected mice. The thymus was the only other organ, besides the brain and liver, to exhibit pathologic changes. Immunohistochemistry with anti-MHV antibodies was performed on thymus sections from MHV-A59-infected β2M(-/-) mice, to rule out possible viral replication in the thymus. However, there was no evidence of viral antigen present in the thymus of MHV-A59-infected β2M(-/-) mice. Control immunohistochemical and in situ hybridization experiments using irrelevant antibodies and probes on adjacent sections were all negative.
Chronic Demyelination in β2M(-/-) Mice Infected with MHV-A59
We showed previously that chronic demyelinating lesions can develop in β2M(-/-) mice; thus CD8+ lymphocytes were not an absolute requirement for MHV-induced demyelination [24]. To further quantify the degree of demyelination in chronically infected β2M(-/-) mice, we examined demyelination using Epon-embedded, 1-μM thick spinal cord sections, stained with toluidine blue and examined by light microscopy. We found only minute foci of demyelination in 14 of 200 quadrants of spinal cord cross sections in 15 β2M(-/-) mice infected with 10 PFU of MHV-A59 (table 1). In the majority of the cases, the lesions were significantly smaller than the ones seen in B6 mice infected with 5,000 PFU of MHV-A59. In some spinal cord sections we found foci of inflammatory cells within the meninges but no evidence of demyelination in adjacent white matter. This degree of demyelination was similar to that observed in B6 mice receiving the same amount of virus (10 PFU) [32]. In B6 mice receiving 5,000 PFU, demyelination was found in all of 5 mice in 65% of the quadrants, which is the usual degree of demyelination produced by MHV-A59 in B6 mice [32].
Pathology of β2M(-/-) Mice with MHV-2
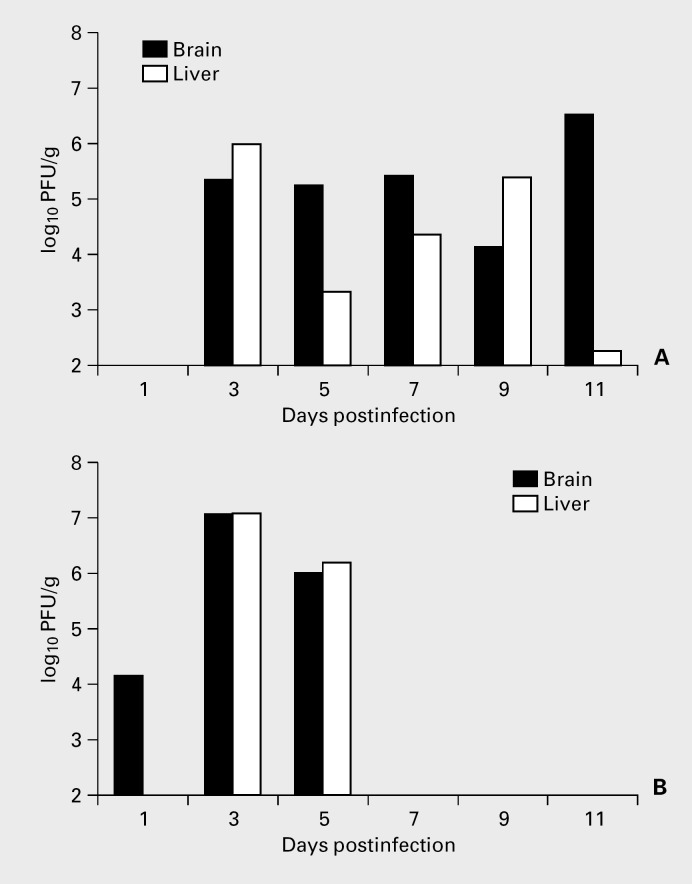
A less neurotropic strain of MHV, MHV-2, which normally does not invade the brain parenchyma in B6 mice, was used in comparison to A59 in order to evaluate the protective effect of CD8+ cells on viral invasion of the brain. Four-week-old β2M(-/-) mice were inoculated i.c. with MHV-2 and analyzed, at various intervals postinfection, for histopathology and viral titers in organs. Histology revealed both acute hepatitis and acute encephalitis. Acute encephalitis was observed in several periventricular areas especially the lateral and 4th ventricles. Mononuclear lymphocytic infiltration involved the choroid plexus, the ependyma and in the surrounding brain parenchyma especially in the brainstem (fig. 2). There was no involvement of other areas of the brain including areas that are typically involved in A59 infection of B6 mice. By contrast, B6 mice infected with MHV-2 showed mostly inflammation of the meninges and choroid plexus while brain parenchyma was only minimally involved. Sustained titers of infectious virus were detected by plaque assay were detected in both brains and livers of β2M(-/-) mice infected with MHV-2. Titers peaked at 3 and 5 days after MHV-2 infection of B6 mice (fig. 3). Neither B6 mice nor 5 β2M(-/-) mice sacrificed 30 days postinfection with 1 LD50 dose of MHV-2, showed any evidence of demyelination in Epon-embedded sections stained with toluidine blue.
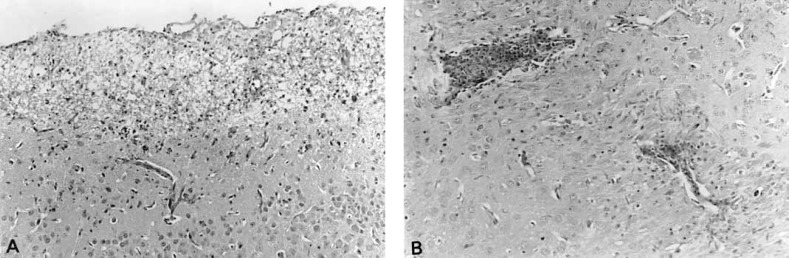
Fig. 2.
Pathologic changes in β2M(-/-) mice infected IC with MHV-2. A Periventricular necrotizing encephalitis. HE. × 100. B Parenchymal brainstem encephalitis with marked perivascular inflammatory infiltration. HE. × 100.
Fig. 3.
Viral titers in organs of MHV-2-infected β2M(-/-) mice (A) compared to MHV-2-infected B6 mice (B). β2M(-/-) mice were infected IC with 5 × 103 PFU of virus and B6 mice were infected IC with 5 × 105 PFU of virus then sacrificed at various intervals postinfection; organs were aseptically removed and viral titers were determined by plaque assay.
Discussion
The ability of mice devoid of β2-microglobulins and MHC class I expression to survive without functional CD8+ cytotoxic T cells [26] provided a unique opportunity to study the contribution of CD8+ cells to the disease process induced in mice by coronavirus infection. Although β2M(-/-) mice may occasionally retain a very small number of functional CD8+ lymphocytes [33], β2M(-/-) mice have been useful for studies of viral pathogenesis. For example, β2M(-/-) mice exhibited delayed clearance and increased mortality following influenza virus challenge [34]. Lymphocytic choriomeningitis virus (LCMV) infection induced a chronic wasting disease in β2M(-/-) mice [35]. In the LCMV model system, CD4+ CTLs may partially compensate for the lack of CD8+ CTLs [36]. However, CD8+ lymphocytes were not required for clearance of vaccinia virus or cytomegalovirus infections [37, 38].
We carefully dissected and compared the disease process in β2M(-/-) mice to that produced in normal B6-susceptible mice in order to further study the role of CD8+ cells in the pathogenesis of MHV infection. β2M(-/-) mice were more susceptible to the virus, showed prolonged course of the disease and had sustained titers of the virus in the brain and liver. The time frame for initiation of disease in the meninges and brain was not significantly different in β2M(-/-) mice compared to B6-infected mice. However, following i.c. inoculation, hepatitis was delayed in onset in β2M(-/-) mice compared to B6 mice. The lack of CD8+ cells had a different effect on various components of the disease process induced by MHV, suggesting that each part of this disease process is independently controlled. In addition, the lack of CD8+ cells increased MHV-2-induced encephalitis without affecting the ability of mice to develop demyelination.
We performed an exhaustive search for additional reservoir of infection in organs other than the brain and liver in order to explain the increased susceptibility of β2M(-/-) mice to MHV infection. We ruled out the thymus as a potential reservoir since there was no evidence of active viral replication in the thymus of infected β2M(-/-) mice or B6 mice by immunohistochemistry and plaque assay. This was consistent with a previous report showing that thymic pathology of lymphocytic depletion in mice infected with MHV is mainly due to apoptosis, possibly mediated by cytotoxic cytokines and not due to active viral replication [39].
Since the extent of hepatitis and the histopathologic changes in the livers of β2M(-/-) mice were similar to B6 mice during acute infection and no additional organs showed any pathologic abnormalities, we concluded that the main cause of the increased virulence and mortality in β2M(-/-) mice was the increased CNS replication. During acute encephalitis in β2M(-/-) mice there was slight change in the anatomical pattern of spread of the virus, in that the thalamus and basal ganglia had more extensive involvement. The amount of involvement of neuronal and glial cells infection was relatively similar in β2M(-/-) mice and B6 mice. The main change in CNS disease in β2M(-/-) mice was in additional cellular reservoirs of infectious virus in cell types that are not typically involved or only minimally infected in MHV-A59 infection of B6 mice. This included perivascular lymphocytes, microglia, and endothelial cells. Thus, CD8+ cells may play a role in clearance of virus from these cell types, causing increased susceptibility of the β2M(-/-) mice to MHV-A59.
We then compared infection of β2M(-/-) mice by two different strains of MHV, which have different neurotropic phenotypes in B6 mice, in order to further assess the role of CD8+ cells in preventing spread of MHV within the brain. MHV-2 and MHV-A59 are closely related strains of MHV exhibiting over 80% homology in nucleotide sequences [Das Sarma et al., manuscript in preparation]. However, the two strains differ in the degree of CNS involvement. While MHV-A59 produces acute encephalitis and chronic demyelination, MHV-2 produced much less encephalitis and no demyelination. We asked whether mice lacking CD8+ show more parenchymal involvement in MHV-2 infection, in order to explore the possible role of CD8+ cells in brain invasion and demyelination. Our results demonstrated that MHV-2 infection in β2M(-/-) mice produced significant brainstem encephalitis without demyelination, while parenchymal involvement in MHV-2-infected B6 mice was minimal. Thus, CD8+ cells may affect the spread of MHV within the brain, but have no effect on demyelination.
As previously demonstrated [24], the extent of demyelination was reduced in β2M(-/-) mice as compared to B6 mice when each type of mice are given 1-2 LD50 dose of virus (10 PFU in β2M(-/-) mice and 3,000 PFU in B6 mice). The reduced rate of demyelination in β2M(-/-) mice may be due to the lower dose of virus used and the finding that the lack of CD8+ cells did not affect the susceptibility of mice to demyelination, despite the increased susceptibility to hepatitis and encephalitis [32].
Studies of β2M(-/-) mice with Theiler's virus, another experimental model system of virus-induced demyelination, showed that CD8+ cells were required for clearance of virus and protection of the brain from infection and subsequent demyelination in a resistant strain [40, 41, 42]. In addition, remyelination was more extensive in the β2M(-/-) mice infected with Theiler's virus which suggested that CD8+ cells may have a role in preventing remyelination [43].
In conclusion, CD8+ cells may have an important role in clearance of virus from infected inflammatory cells, endothelial cells and microglial cells, during acute MHV infection of the brain. However, CD8+ cells had less effect on viral clearance from primary CNS cells such as neurons astrocytes and oligodendrocytes which probably use different mechanisms of clearance. Failure to achieve complete viral clearance from the CNS, especially in oligodendrocytes and astrocytes, may result in viral persistence and chronic demyelination. Our studies showed no evidence of a direct role of CD8+ cells in chronic demyelination.
Acknowledgement
Supported in part by National Multiple Sclerosis Society grants RG-2615A1/2 and RG-2585A/1. Joyce A. Haluskey and Qian Wang provided technical help.
References
- 1.McIntosh K. Coronaviruses: A comparative review. Curr Top Microbiol Immunol. 1974;63:80–129. [Google Scholar]
- 2.Wege H, Siddell S, ter Meulen V. The biology and pathogenesis of coronaviruses. Adv Virol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- 3.Lavi E, Weiss SR, Coronaviruses . In: Clinical and Molecular Aspects of Neurotropic Viral Infections. Gilden DH, Lipton HL, editors. Boston, Kluwer: Dev Med Virol; 1989. pp. pp 101–139. [Google Scholar]
- 4.Weiner LP. Pathogenesis of demyelination induced by a mouse hepatitis virus (JHM virus) Arch Neurol. 1973;28:298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- 5.Stohlman SA, Weiner LP. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981;31:38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- 6.Knobler RL, Haspel MV, Oldstone MBA. Mouse hepatitis virus type 4 (JHM strain)-induced fatal central nervous system disease. 1. Genetic control and the murine neurone as the susceptible site for disease. J Exp Med. 1981;153:832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen O, Durge R, Percy D, Dales S. In vivo and in vitro models of demyelinating disease: Endogenous factors influencing demyelinating disease caused by mouse hepatitis virus in rats and mice. Infect Immun. 1982;37:1248–1260. doi: 10.1128/iai.37.3.1248-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier MJ, Lewicki HA, Talbot PJ, Knobler RL. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984;132:261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barthold SW, Smith AL. Mouse hepatitis virus S in weanling Swiss mice following intranasal inoculation. Lab Anim Sci. 1983;33:355–360. [PubMed] [Google Scholar]
- 10.Lavi E, Gilden DH, Wroblewska Z, Rorke LB, Weiss SR. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984;34:597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- 11.Perlman S, Jacobsen G, Olson AL, Afifi A. Identification of the spinal cord as a major site of persistence during chronic infection with a murine coronavirus. Virology. 1990;175:418–426. doi: 10.1016/0042-6822(90)90426-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houtman JJ, Fleming JO. Pathogenesis of mouse hepatitis virus-induced demyelination. J Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- 13.Lane TE, Buchmeier MJ. Murine coronavirus infection: A paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FI, Stohlman SA, Fleming JO. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990;30:31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzumura A, Lavi E, Weiss SR, Silberberg DH. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986;232:991–993. doi: 10.1126/science.3010460. [DOI] [PubMed] [Google Scholar]
- 16.Suzumura A, Lavi E, Bhat S, Murasko DM, Weiss SR, Silberberg DH. Induction of glial cell MHC antigen expression in neurotropic coronavirus infection: Characterization of the H-2 inducing soluble factor elaborated by infected brain cells. J Immunol. 1988;140:2068–2072. [PubMed] [Google Scholar]
- 17.Lavi E, Suzumura A, Lampson LA, et al. Expression of MHC class I genes in mouse hepatitis virus (MHV-A59) infection and in multiple sclerosis. Adv Exp Med Biol. 1987;218:219–222. doi: 10.1007/978-1-4684-1280-2_26. [DOI] [PubMed] [Google Scholar]
- 18.Lavi E, Suzumura A, Murray EM, Silberberg DH, Weiss SR. Induction of MHC class I antigens on glial cells is dependent on persistent mouse hepatitis virus infection. J Neuroimmunol. 1989;22:107–111. doi: 10.1016/0165-5728(89)90040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gombold JL, Weiss SR. Mouse hepatitis virus A59 increases steady-state levels of MHC mRNAs in primary glial cell cultures and in the murine central nervous system. Microb Pathog. 1992;13:493–505. doi: 10.1016/0882-4010(92)90015-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houtman JJ, Fleming JO. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 21.Fleming JO, Wang FI, Trousdale MD, Hinton DR, Stohlman SA. Interaction of immune and central nervous systems: Contribution of anti-viral Thy-1+ cells to demyelination induced by coronarvirus JHM. Reg Immunol. 1993;5:37–43. [PubMed] [Google Scholar]
- 22.Sussman MA, Shubin RA, Kyuwa S, Stohlman SA. Cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J Virol. 1989;63:3051–3056. doi: 10.1128/jvi.63.7.3051-3056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin MT, Stohlman SA, Hinton DR. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gombold JL, Sutherland RM, Lavi E, Paterson Y, Weiss SR. Mouse hepatitis virus A59-induced demyelination can occur in the absence of CD8+ T cells. Microb Pathog. 1995;18:211–221. doi: 10.1016/S0882-4010(95)90058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutherland RM, Chua M-M, Lavi E, Weiss SR, Paterson Y. CD4+ and CD8+ T cells are not major effectors of mouse hepatitis virus A59-induced demyelinating disease. J Neurovirol. 1997;3:225–228. doi: 10.3109/13550289709018297. [DOI] [PubMed] [Google Scholar]
- 26.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 27.Keck JG, Matsushira GK, Makino S, et al. In vivo RNA-RNA recombination of coronavirus in mouse brain. J Virol. 1988;62:1810–1813. doi: 10.1128/jvi.62.5.1810-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavi E, Fishman SP, Highkin MK, Weiss SR. Limbic encephalitis following inhalation of murine coronavirus MHV-A59. Lab Invest. 1988;58:31–36. [PubMed] [Google Scholar]
- 29.Lavi E, Strizki JM, Ulrich AM, et al. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 30.Lee VM-Y, Page CD, Wu HL, Schlaepfer WW. Monoclonal antibodies to gel excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984;42:25. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- 31.Lavi E, Murray EM, Makino S, Stohlman SA, Lai MM, Weiss SR. Determinants of coronavirus MHV pathogenesis are localized to 3′ portions of the genome as determined by ribonucleic acid-ribonucleic acid recombination. Lab Invest. 1990;62:570–578. [PubMed] [Google Scholar]
- 32.Leparc-Goffart I, Hingley ST, Chua M-M, Jiang X, Lavi E, Weiss SR. Altered pathogenesis phenotypes of murine coronavirus MHV-A59 are associated with a Q159L amino acid substitution in the receptor binding domain of the spike protein. Virology. 1997;239:1–10. doi: 10.1006/viro.1997.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roullet E, Assuerus V, Gozlan J, et al. Cytomegalovirus multifocal neuropathy in AIDS: Analysis of 15 consecutive cases. Neurology. 1994;44:2174–2182. doi: 10.1212/wnl.44.11.2174. [DOI] [PubMed] [Google Scholar]
- 34.Bender BS, Croghan T, Zhang L, Small PAJ. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty PC, Hou S, Southern PJ. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J Neuroimmunol. 1993;46:11–17. doi: 10.1016/0165-5728(93)90228-q. [DOI] [PubMed] [Google Scholar]
- 36.Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. LCMV-specific, class II-restricted cytotoxic T cells in beta-2-microglobulin-deficient mice. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 37.Spriggs MK, Koller BH, Sato T, et al. Beta-2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polic B, Jonjic S, Pavic I, Crukovic I, Zorica I, Hengel H, Lucin P, Koszinoski UH. Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J Gen Virol. 1996;77:217–225. doi: 10.1099/0022-1317-77-2-217. [DOI] [PubMed] [Google Scholar]
- 39.Lee SK, Youn HY, Hasegawa A, Nakayama H, Goto N. Apoptotic changes in the thymus of mice infected with mouse hepatitis virus, MHV-2. J Vet Med Sci. 1994;56:879–882. doi: 10.1292/jvms.56.879. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez M, Dunkel AJ, Thiemann RL, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in beta-2-microglobulin. J Immunol. 1993;151:266–276. [PubMed] [Google Scholar]
- 41.Pullen LC, Miller SD, Dal Canto MC, Kim BS. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur J Immunol. 1993;23:2287–2293. doi: 10.1002/eji.1830230935. [DOI] [PubMed] [Google Scholar]
- 42.Fiette L, Aubert C, Brahic M, Rossi CP. Theiler's virus infection of beta-2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller DJ, Rivera-Quinones C, Njenga MK, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in beta-2-microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15:8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]