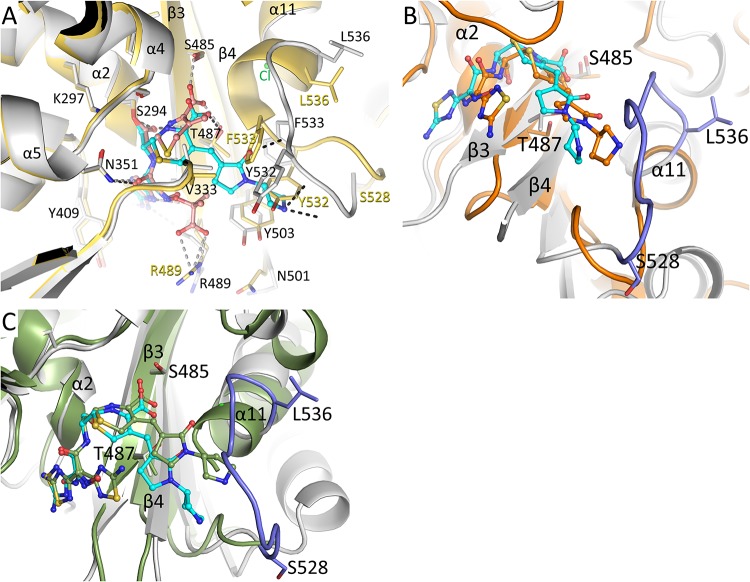
FIG 3.
Structural comparison of the ceftobiprole-PBP3 structure. (A) Superposition of the ceftobiprole- and ceftazidime-bound PBP3 structures. The root mean square deviation (RMSD) of the 466 aligned C-α atoms is 1.00 Å (the ceftazidime-PaPBP3 structure has PDB accession number 3PBO [4]). The PBP3 protein residues from the ceftobiprole and ceftazidime complexes are colored gray and yellow, respectively. The cephalosporin ligands are both shown in ball-and-stick representation, ceftobiprole with cyan carbon atoms and ceftazidime with orange carbon atoms. Hydrogen bonds of ceftobiprole and ceftazidime with the protein are shown as black and gray dashed lines, respectively. Residues that differ significantly between the two structures have the two different conformations labeled in black or yellow; other residues have a single black label. Key secondary structural elements are also labeled. (B) Superposition of the ceftobiprole-bound PaPBP3 and ceftobiprole-bound Staphylococcus aureus PBP4 (PDB accession number 5TXI [14]) structures. The PBP4 protein is colored orange, as are the carbon atoms of its bound ceftobiprole; the PBP3-ceftobiprole atoms are colored as in panel A except for residues 528 to 536, which are colored light blue. The following residues were used for superpositioning of the active sites: PBP3 residues 290 to 307, 345 to 360, 406 to 409, 484 to 488, and 505 to 511 were superimposed on PBP4 residues 71 to 88, 135 to 150, 179 to 182, 259 to 263, and 268 to 274, respectively; the RMSD for 50 C-α atoms was 1.34 Å. (C) Superposition of the ceftobiprole-bound PaPBP3 and ceftobiprole-bound Staphylococcus aureus PBP2a (PDB accession number 4DKI [15]) structures. The PBP2a protein is colored green, as are the carbon atoms of its bound ceftobiprole; the PBP3-ceftobiprole atoms are colored as in panel B. The following residues were used for superpositioning of the active sites: PBP3 residues 290 to 307, 340 to 360, 406 to 409, 482 to 488, and 503 to 510 were superimposed on PBP2a residues 399 to 416, 453 to 473, 518 to 521, 595 to 601, and 613 to 620, respectively; the RMSD for 58 C-α atoms was 1.06 Å.