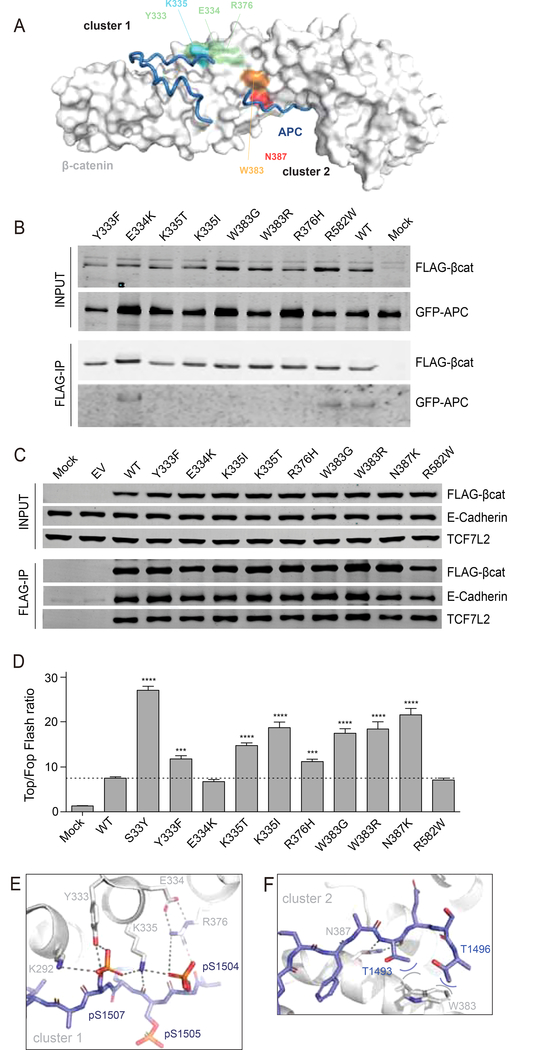
Figure 7. Increased signaling and selective reduction of APC binding for most armadillo repeat 5/6 variants.
(A) Surface representation of β-catenin in grey with bound APC as tube representation in blue. K335 in cluster-1 is colored light-blue, with surrounding residues Y333, E334 and R376 in light-green. N387 in cluster-2 is colored red, while the other commonly mutated W383 is colored orange. (B) Co-transfection of indicated FLAG-tagged β-catenin variants with GFP-APC in HEK293 cells. Following FLAG-IP, most of the commonly observed armadillo repeat 5/6 variants associate much weaker to GFP-APC. (C) FLAG-tagged β-catenin variants were transfected in HCT116 cells. Following FLAG-IP all variants are shown to bind equally to endogenous TCF7L2 and E-cadherin. (D) A Top-Fopflash β-catenin reporter assay in HEK293 cells shows that all armadillo repeat 5/6 variants that bind weaker to APC show a 1.5–3 fold increased signaling compared with wild-type β-catenin. Top-Fopflash ratios are shown. Experiments were performed in triplo and repeated twice. Data are presented as mean ± SD. ***, P<0.001; ****, P<0.0001. (E) Details of the cluster-1 complex, with β-catenin as grey cartoon and APC as blue stick representation. A six-residue ion-pair network is formed between E334-R376;R376-pS1504;pS1504-K335;K335-pS1507;pS1507-K292. In fact, the network is even bigger and extends beyond K292 (not shown). Hydrogen bonds are indicated with dashed lines. (F) Details of the cluster-2 complex. W383 packs against the hydrophobic methyl-groups (blue carbons) of the sidechains of T1493 and T1496 and so forms favorable van-der-Waals interactions (curved lines).