Figure 1. Activation of the dynein transport machinery.
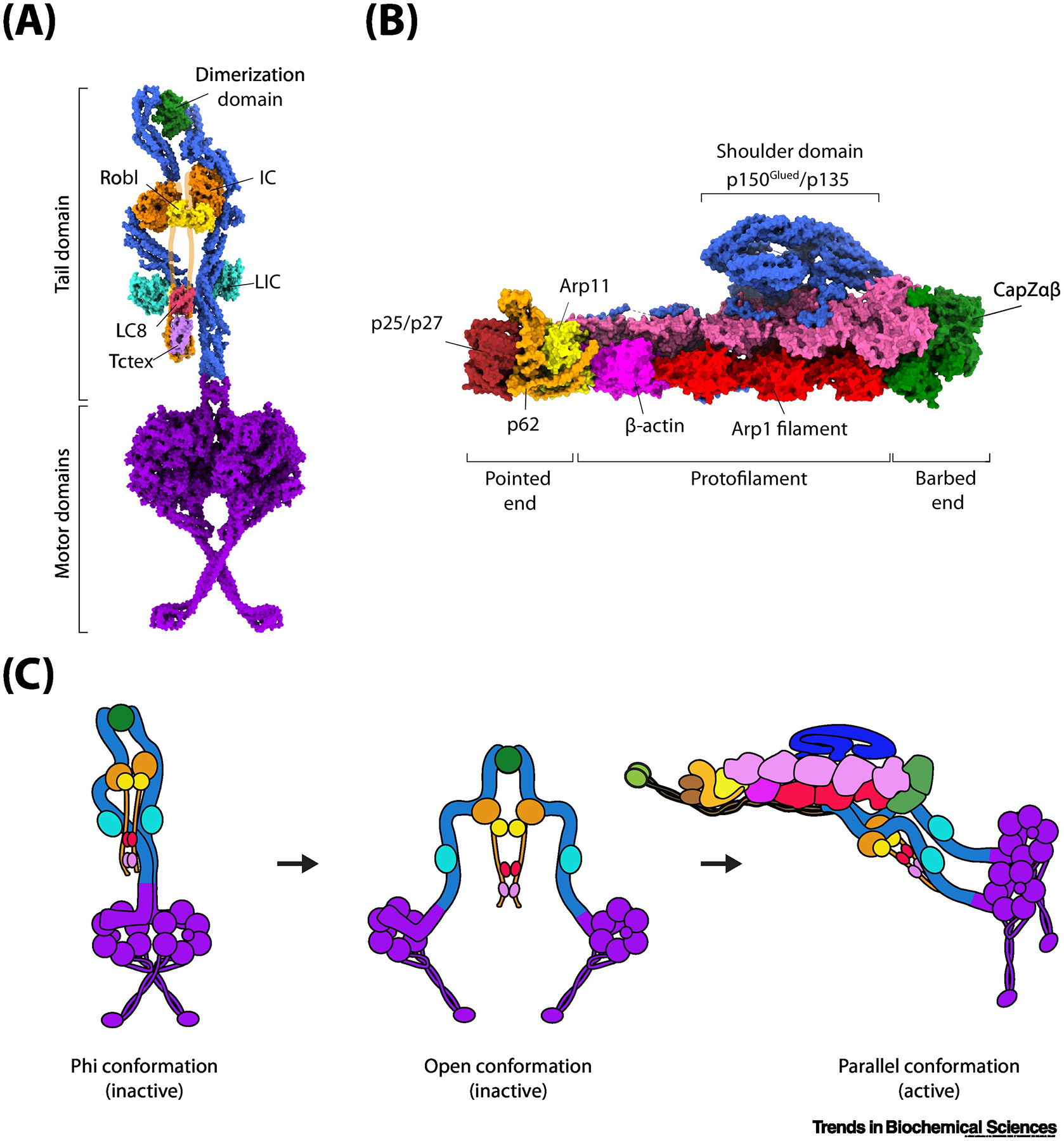
(A) The architecture of the dynein complex in the autoinhibited (phi) conformation (PDB accession: 5NVU). The N-terminal dimerization domain links the two DHCs (blue) together. The intermediate chains (ICs) are held together through an N-terminal unstructured region (purported location shown in orange outline) through homodimers of Robl, LC8, and Tctex. The light intermediate chains (LICs, cyan) bind mid-way along the tail domain. The motor domains (purple) form a stacked structure with the coiled-coil stalks crossed. (B) The architecture of the dynactin complex (PDB accession: 5ADX). The two Arp1 filaments (red and magenta) form the core of the dynactin structure. The barbed end is capped by CapZαβ (green) and the pointed end is capped by p25/p27 (dark red), p62 (orange), and Arp11 (yellow). Atop the Arp1 filaments sits a large shoulder-like domain consisting of p150Glued/p135 (blue). p150Glued also consists of a large coiled-coil projection terminating with the CAP-Gly domain (not shown). (C) Isolated dynein forms an autoinhibited phi conformation through self-dimerization of the motor domains and only weakly interacts with a MT. Disruption of the phi interaction sites separates the two motor domains, but dynein is unable to move processively in this open conformation because motor domains point towards each other. Binding to dynactin and a cargo adaptor orients the motor domains into a parallel conformation for processive movement along MTs.
