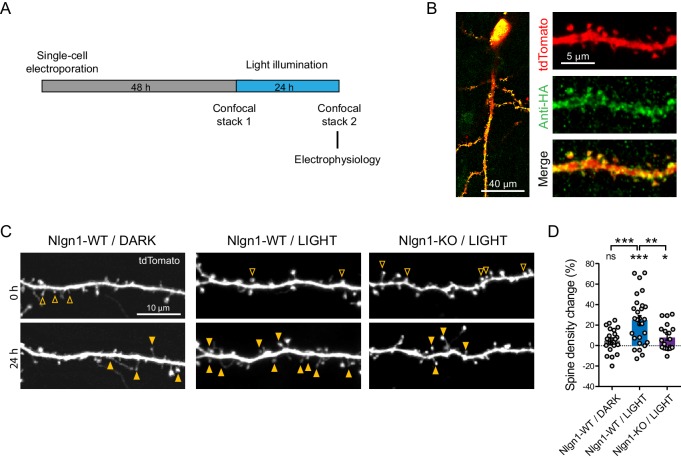
Figure 2. Optogenetic stimulation of optoFGFR1 increases dendritic spine density in a Nlgn1-dependent manner.
(A) Experimental procedure to investigate the effect of the optogenetic stimulation of optoFGFR1 on spine density and synaptic transmission. CA1 neurons in organotypic slices from WT or Nlgn1 KO mice were electroporated at DIV 3–5 with tdTomato and HA-tagged optoFGFR1. Two days later, they were either stimulated with alternating blue light for 24 hr or kept in the dark, and processed for imaging or electrophysiology. (B) Confocal images of CA1 neurons and dendritic segments showing tdTomato (red) and anti-HA immunostaining (green). (C) Confocal images of apical dendrites from electroporated neurons before (0 hr) and 24 hr after light activation of optoFGFR1. Control slices did not receive light, or received light but were from the Nlgn1 KO background. Solid arrowheads point to spines which have appeared, and empty arrowheads to spines which have disappeared in the time interval. (D) Normalized spine density for each condition (n = 19–28 dendrites from N = 5–7 cells). Change in spine density was assessed for each condition using paired t-test (***p<0.001, *p<0.05, ns: not significant). Spine density change was compared across conditions using a one-way ANOVA followed by Tukey’s multiple comparison test (***p<0.001, **p<0.01).