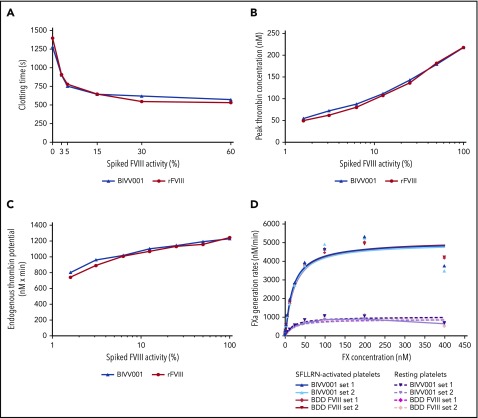
Figure 5.
In vitro hemostatic potential of BIVV001. (A) The effect of BIVV001 on whole-blood clotting time was assessed by using ROTEM and the extrinsically activated test with tissue factor assay. Whole blood from a blood donor with HemA was spiked with increasing concentrations of BIVV001 or rFVIII to obtain FVIII activities of 0% to 60% (based on a 1-stage activity assay). The procoagulant activity of BIVV001 and rFVIII was assessed by measuring peak thrombin concentration (B) and endogenous thrombin potential (C) using a thrombin generation assay. Congenitally FVIII-deficient plasma was spiked with BIVV001 and rFVIII (FVIII activities of 1.6%-100%). Representative curves are shown for ROTEM and thrombin generation assay. (D) The procoagulant properties of BIVV001 and BDD FVIII as precursors to the Xase complex (FVIIIa-FIXa) were assessed using an FXa generation assay, as previously described.24,30,31 Xase complex Km values were calculated by measuring the conversion of FX to FXa, by using an FXa-specific chromogenic assay. Xase activity was assessed in the presence of Ca2+ and resting (R) or SFLLRN-activated human platelets (in duplicate).