Abstract
Background
Minimally invasive surgery (MIS) is an accepted surgical technique for the treatment of a variety of benign diseases. Presently, the use of MIS in patients with cancer is progressing. However, the role of MIS in children with solid neoplasms is less clear than it is in adults. Although the use of diagnostic MIS to obtain biopsy specimens for pathology is accepted in paediatric surgical oncology, there is limited evidence to support the use of MIS for the resection of malignancies. This review is the second update of a previously published Cochrane review.
Objectives
To ascertain differences in outcome between the minimally invasive and open surgical approaches for the treatment of solid intra‐abdominal or intra‐thoracic neoplasms in children. The primary outcomes of interest are OS, EFS, port‐site metastases and recurrence rate; the secondary outcome of interest is surgical morbidity.
Search methods
We searched CENTRAL (The Cochrane Library 2014, Issue 1), MEDLINE/PubMed (from 1966 to February 2014) and EMBASE/Ovid (from 1980 to February 2014) to identify relevant studies. In addition, we searched reference lists of relevant articles and reviews and the conference proceedings of the International Society for Paediatric Oncology and the American Society of Clinical Oncology from 2003 to 2013. On 1 May 2014 we scanned the ISRCTN Register (on www.controlled‐trials.com), the National Institutes of Health register (on www.controlled‐trials.com and www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (on www.apps.who.int/trialsearch) for ongoing trials.
Selection criteria
Randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing MIS to open surgery for the treatment of solid intra‐thoracic or intra‐abdominal neoplasms in children (aged 0 to 18 years) were considered for inclusion.
Data collection and analysis
Two authors performed the study selection independently.
Main results
The literature search retrieved 542 references. After screening the titles and abstracts we excluded 534 references which clearly did not meet the inclusion criteria. We assessed eight full text studies for eligibility and all of these studies were excluded from the review because they were not RCTs or CCTs. These excluded studies included case series, retrospective chart reviews and retrospective cohort studies. The scanning of reference lists and conference proceedings did not identify any additional studies and no (ongoing trials) were identified by the searches of trial registries. No studies that met the inclusion criteria of this review were identified
Authors' conclusions
No RCTs or CCTs evaluating MIS for the treatment of solid intra‐thoracic or intra‐abdominal neoplasms in children could be identified. The current evidence base informing the use of MIS in children with solid abdominal and thoracic neoplasms is based on other study designs like case reports, retrospective chart reviews and cohort studies and should be interpreted with caution. Thus there is insufficient evidence to allow any definitive conclusions regarding the use of MIS in these patients. High quality RCTs comparing MIS to open surgery are required. To accomplish this, centres specialising in MIS in children should collaborate.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Infant, Newborn; Abdominal Neoplasms; Abdominal Neoplasms/surgery; Laparoscopy; Laparotomy; Thoracic Neoplasms; Thoracic Neoplasms/surgery; Thoracoscopy; Thoracotomy
Plain language summary
Minimally invasive surgery compared to open surgery for the treatment of solid tumours located in the chest or the abdomen of children
Minimally invasive surgery (MIS) is an upcoming new surgical technique. MIS is done through one or more small incisions using a laparoscope or thoracoscope (a thin flexible tube containing a video camera) and surgical instruments. MIS can be used as a diagnostic instrument (i.e. to retrieve tissue samples for a biopsy) and is also used for the resection (i.e. to remove by surgery) of tumours (a lump or growth in a part of the body that is formed from abnormal cells). There is limited experience with the use of MIS for the resection of solid tumours in the chest or abdomen in children.
This systematic review focused on randomised controlled trials (RCTs) and controlled clinical trials (CCTs). The authors could not identify any RCTs or CCTs RCTs on this subject. Thus there is insufficient evidence to allow any definitive conclusions regarding the therapeutic use of MIS in children with solid tumours in the chest or abdomen. More high quality RCTs are needed.
Background
Minimally invasive surgery (MIS) is an accepted surgical technique for the treatment of a variety of benign diseases. After the introduction of laparoscopic cholecystectomy, other surgical procedures such as appendectomy, fundoplication, splenectomy and nephrectomy were soon performed using MIS (Bax 2005; Georgeson 2000; Georgeson 2003; Johnson 1997; Schmidt 2007; Ure 2000). MIS showed short‐term postoperative advantages compared to open surgery including less pain, a shorter duration of postoperative ileus and better pulmonary function, all leading to a more rapid recovery and shorter hospital stay (Bax 2005; Leung 2004; Milsom 1998).
In patients with cancer, the use of MIS is progressing. Although randomised studies in adult cancer patients have increasingly been published, in general it remains controversial whether MIS is appropriate for the resection of many types of neoplasms with regard to long‐term survival rates. Although prospective studies comparing laparoscopy with laparotomy in adults with colorectal cancer initially showed short‐term postoperative advantages for MIS (Leung 2004; Milsom 1998), the development of port‐site metastases in these patients (Berends 1994; Lacy 2002), led to concerns regarding the safety of tumour clearance through port sites and long‐term survival (Lacy 2002). It became clear that any initial excitement had to await the results of randomised controlled trials (RCTs) that reported on longer‐term follow‐up data including overall survival (OS) and event‐free survival (EFS). Lacy 2002 showed that the laparoscopic approach might have survival advantages over the conventional open approach, but this difference was not statistically significant (Lacy 2002). A meta‐analysis by Liang et al showed that compared to open resection for colorectal cancer the laparoscopic approach did not increase the rates of overall recurrence, local recurrence, distant metastases and port or wound‐site recurrences (Liang 2008). In a trial of 1248 adult patients randomly assigned to either laparoscopic or open resection for colon cancer, a small difference in disease‐free survival at three years in favour of open colectomy could not be ruled out (Buunen 2009).
The role of MIS in children with solid neoplasms is less clear than it is in adults. However, there is growing experience in the use of MIS as a feasible technique to resect malignancies in children (Iwanaka 2004; Saenz 1997; Spurbeck 2004). Although the existing studies are all very positive about the growing role MIS may play in treating paediatric solid tumours, most of the evidence is based on results from studies in adults. The extrapolation of results from studies in adults to children is controversial due to differences in tumour biology in children, and in the treatment and prognosis of paediatric tumours. To date MIS seems mostly to have a reliable diagnostic use in children (Metzelder 2007). Up to date, therapeutic MIS is increasingly used to treat solid intra‐thoracic and intra‐abdominal neoplasms in children without (extensive) evidence supporting this practice (Al‐Shanafey 2008; Castilho 2002; Leclair 2007; Warmann 2003). This systematic review is the second update of the first systematic review evaluating the state of evidence on this topic, focusing on randomised controlled trials (RCTs) and controlled clinical trials (CCTs) (De Lijster 2010; De Lijster 2012).
Objectives
Primary objective
To ascertain differences in outcome between the minimally invasive and open surgical approaches for the treatment of solid intra‐abdominal or intra‐thoracic neoplasms in children. The primary outcomes of interest are OS, EFS, port‐site metastases and recurrence rate.
Secondary objective
To ascertain differences in surgical morbidity between the minimally invasive and open surgical approaches.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing minimally invasive surgery (MIS) and open surgery for the treatment of intra‐thoracic or intra‐abdominal solid neoplasms were considered for inclusion.
Types of participants
Children (aged 0 to 18 years at diagnosis) with solid intra‐thoracic or intra‐abdominal neoplasms who were treated with MIS or open surgery (irrespective of previous therapy) were considered for inclusion.
Types of interventions
Studies that compared MIS (e.g. laparoscopy or thoracoscopy) to open surgery (e.g. laparotomy or thoracotomy) were considered for inclusion.
Types of outcome measures
Primary outcomes
OS: defined as the time from surgery to death from any cause.
EFS: as defined by the authors of the original study.
Port‐site metastases: defined as tumour recurrence in trocar sites or surgical wounds.
Recurrence rate: defined as the rate of either local or distant recurrence.
Secondary outcome
Surgical morbidity, with regard to length of operation, intra‐operative blood loss, postoperative complications (such as wound infection and bleeding), restart of oral intake, pain score, and length of hospital stay.
Search methods for identification of studies
We searched the following electronic databases: CENTRAL (The Cochrane Library 2014, Issue 1), MEDLINE/PubMed (from 1966 to 18 February 2014) and EMBASE/Ovid (from 1980 to 18 February 2014). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text word terms) are reported in the Appendices (Appendix 1; Appendix 2; Appendix 3).
We searched the reference lists of relevant articles and reviews to identify additional studies. We also scanned the conference proceedings of the International Society for Paediatric Oncology (SIOP) and American Society of Clinical Oncology (ASCO) from 2003 to 2013, if available electronically and otherwise by handsearching (see Appendix 4 for the search strategy used for the latest update). We searched for ongoing studies in the ISRCTN Register (on www.controlled‐trials.com), the National Institutes of Health (NIH) register (on www.controlled‐trials.com and www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (on www.apps.who.int/trialsearch) (all searched on 1 May 2014; see Appendix 5 for the search strategies used in the latest update). We imposed no language restriction.
Data collection and analysis
Study identification
After employing the search strategy described previously, two authors independently screened titles and abstracts to identify studies meeting the inclusion criteria. Discrepancies were resolved by consensus; no third party arbitration was needed. Any study seemingly meeting the inclusion criteria on the grounds of title, abstract, or both, was obtained in full for closer inspection.
Risk of bias in included studies
If eligible studies had been identified, two authors would have independently assessed the risk of bias in these studies according to the criteria of the Cochrane Childhood Cancer Group. However, since no eligible studies were identified the assessment of the risk of bias was not applicable.
Data extraction
Since no eligible studies were identified, data extraction by two independent authors using a standardised form could not be performed.
Data analyses
No eligible studies were identified. As a result, data analyses could not be performed.
Results
Description of studies
After performing the searches of the electronic databases of CENTRAL, MEDLINE/PubMed AND EMBASE/Ovid, we identified 542 references (including 129 in the first update and 164 in the second update). Initial screening of the titles and/or abstracts excluded 534 references which clearly did not meet all criteria for considering studies for this review. We obtained eight articles in full. However, these studies were not randomised controlled trials (RCTs) or controlled clinical trials (CCTs) evaluating MIS in children with solid intra‐thoracic or intra‐abdominal neoplasms and were thus not eligible for inclusion in this review (See Figure 1 and the Characteristics of excluded studies table).
1.
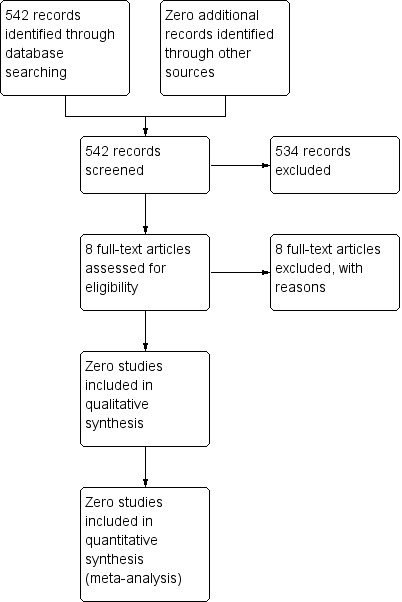
Study flow diagram.
Scanning the reference lists of relevant studies and reviews, and scanning the conference proceedings of SIOP and ASCO, did not identify any other eligible studies. Scanning the ongoing trials databases did not identify any eligible (ongoing) studies.
In summary, our search did not identify any eligible RCTs or CCTs evaluating MIS for the treatment of solid intra‐thoracic or intra‐abdominal neoplasms in children.
Risk of bias in included studies
Since no eligible studies were identified, the assessment of the risk of bias in included studies was not applicable.
Effects of interventions
Since no eligible studies were identified, the effects of MIS versus open surgery for the treatment of solid intra‐thoracic or intra‐abdominal neoplasms in children remain unclear.
Discussion
MIS is well established for many operative procedures in adults, including biopsies to confirm a diagnosis, staging of malignancies and surgical treatment of malignancies (Buunen 2009; Spurbeck 2004). The use of MIS for the evaluation and treatment of solid neoplasms in children has increased rapidly over the last decade (Al‐Shanafey 2008; Castilho 2002; Duarte 2009; Leclair 2007; Metzelder 2007; Sailhamer 2003; Spurbeck 2004; Varlet 2009; Warmann 2003). This is the second update of the Cochrane systematic review evaluating the current state of evidence on the therapeutic use of MIS in children with solid intra‐thoracic or intra‐abdominal tumours (De Lijster 2010; De Lijster 2012).
To evaluate the role of MIS for the treatment of solid intra‐thoracic and intra‐abdominal childhood tumours adequately the best study design, with the highest level of evidence, is a RCT. Unfortunately, we could not identify any RCTs. We were also unable to identify any eligible CCTs.
Even though results from adult RCTs in patients with colon cancer are promising (Buunen 2009; Lacy 2002; Leung 2004), the extrapolation of results from studies in adults to children is not possible, due to the different tumour biology of adult and paediatric malignancies, and the differences in the therapy and prognosis of cancer in children. For example, the short‐term advantages of MIS over open surgery in adults might be less in children, since children recover faster than adults after an open surgical procedure. RCTs in children with solid intra‐thoracic or intra‐abdominal neoplasms are therefore needed. Ehrlich 2002 started a RCT to evaluate the role of MIS in children with cancer, but unfortunately this study failed. This study failed because of failure to accrue patients, lack of surgical expertise with MIS procedures within surgical teams and preconceived surgeon bias toward MIS or open surgery. When using MIS as a new technique, most complications occur during the learning curve (Song 2009); only with experience can the constraints of MIS be overcome. However, in the paediatric field the number of patients is limited, making the learning curve longer. Despite the small size of the abdominal cavity in children, which can restrict adequate visualisation, Iwanaka et al demonstrated that the laparoscopic resection of solid tumours, such as neuroblastomas, is feasible (Iwanaka 2004). Another difficulty in comparing MIS with open surgery in the paediatric oncologic population is the ongoing progress with different pre‐ and postoperative chemotherapy and radiotherapy treatments. Long‐term follow‐up results (survival) will therefore be difficult to compare, unless the operative technique becomes part of the trial.
Even though RCTs are the highest level of evidence, it should be recognised that data from non‐randomised studies on the use of MIS in different types of solid intra‐thoracic and intra‐abdominal childhood tumours are available (Al‐Shanafey 2008; Castilho 2002; Duarte 2009; Iwanaka 2004; Leclair 2007; Metzelder 2007; Sailhamer 2003; Shanberg 2006; Spurbeck 2004; Varlet 2009; Warmann 2003). Although the results of these studies are promising they should be interpreted with caution due to the biases associated with non‐randomised study designs. Most of these studies included retrospective cohort studies and only a few prospective cohort studies were performed. Duarte 2009, for example, concluded that laparoscopic nephrectomy for Wilms' tumour is a feasible and safe procedure in the short term in a selected group of children after chemotherapy. Duarte 2009 reported that MIS has important advantages including a shorter hospital stay and more cosmetically acceptable incisions (Duarte 2009).
However, the role of MIS as a primary curative technique compared to open surgery in children with solid intra‐thoracic or intra‐abdominal tumours can only be adequately determined through evaluation within prospective RCTs. Hence, surgeons have to realise that currently the use of MIS for solid intra‐thoracic or intra‐abdominal neoplasms in children should be regarded as an experimental treatment that should only be performed in the context of a clinical trial.
Authors' conclusions
Implications for practice.
No randomised controlled trials (RCTs) and controlled clinical trials (CCTs) evaluating the role of minimally invasive surgery (MIS) in solid intra‐thoracic or intra‐abdominal neoplasms in children could be identified. The current evidence base informing the use of MIS in children with solid abdominal and thoracic neoplasms is based on other study designs such as case series, retrospective chart reviews and cohort studies and should be interpreted with caution. Thus there is insufficient evidence to allow any definitive conclusions regarding overall survival, event‐free survival, port‐site metastases, recurrence rate and surgical morbidity associated with the use of MIS in these patients. MIS for solid intra‐thoracic or intra‐abdominal neoplasms in children currently must be regarded as an experimental treatment that should only be performed in the context of a clinical trial. The role of MIS for paediatric solid tumours therefore remains a challenge and has yet to be defined.
Implications for research.
High quality RCTs comparing MIS to open surgery are required. These RCTs should be performed in homogeneous study populations (for example, with regard to tumour type and stage of disease). They should have a long‐term follow up and the number of included patients should be sufficient to obtain the power needed for the results to be reliable. To obtain adequate numbers of patients, centres specialising in MIS in children should collaborate.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2014 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in the review. As a result the conclusions have not changed. |
| 30 June 2014 | New search has been performed | The search for eligible studies was updated to 18 February 2014. |
Acknowledgements
Elvira van Dalen and Leontien Kremer, the Co‐ordinating Editors of the Cochrane Childhood Cancer Group, are co‐authors of this review and therefore they could not act as the Co‐ordinating Editor for this review. Aleida Postma (Department of Paediatric Oncology of the University Medical Center Groningen and University of Groningen, Beatrix Children's Hospital, Groningen, the Netherlands) was willing to take over this task, for which we would like to thank her. We would also like to thank Edith Leclercq, the Trials Search Co‐ordinator of the Cochrane Childhood Cancer Group, for running the search strategy in the different databases and providing us with the titles and abstracts of possible eligible studies. Rosemarijn Bergevoet was an author on the original version of the review and the first update, her work is greatly appreciated. The editorial base of the Cochrane Childhood Cancer Group is funded by Kinderen Kankervrij (KIKA).
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. For thedifferent surgical interventions the following text words were used:
(MIS OR Minimally Invasive Surgery OR Minimal Access Surgical Procedures OR Minimal Surgical Procedures OR Minimally Invasive Surgical Procedures OR Minimal Surgical Procedure OR minimally invasive surgical procedure OR minimal access surgical procedure OR laparoscopy OR laparoscopies OR laparoscope OR laparoscopes OR laparos* OR laparoscopic OR Celioscopy OR Celioscopies OR Peritoneoscopy OR Peritoneoscopies OR Laparoscopic Surgical Procedure OR Laparoscopic Surgical Procedures OR Laparoscopic Surgery OR Laparoscopic Surgeries OR thoracoscopy OR thoracoscopies OR thoracoscope OR thoracoscopes OR thoracos* OR thoracoscopic OR Pleural Endoscopies OR Pleural Endoscopy OR Pleuroscopy OR Pleuroscopies OR Thoracoscopic Surgical Procedure OR Thoracoscopic Surgeries OR Thoracoscopic Surgery OR Thoracoscopic Surgical Procedures OR VATS OR VATSS OR Video‐Assisted Thoracic Surgeries OR Video‐Assisted Thoracic Surgery OR Video Assisted Thoracic Surgery OR Video‐Assisted Thoracoscopic Surgery OR Video Assisted Thoracoscopic Surgery OR Video‐Assisted Thoracoscopic Surgeries OR videolaparoscopy OR videolaparoscopies):ti,ab,kw
2. Forchildhood cancer the following text words were used:
(lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR primitive neuroectodermal tumors OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm*):ti,ab,kw
3. Forchildren the following text words were (will be) used:
(infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy):ti,ab,kw
Final search 1 AND 2 AND 3
The search will be performed in title, abstract or keywords
[*=zero or more characters]
Appendix 2. Search strategy for PubMed
1. For thedifferent surgical interventions the following MeSH headings and text words were used:
(MIS OR Surgical Procedures, Minimally Invasive OR Minimally Invasive Surgery OR Procedures, Minimal Access Surgical OR Procedures, Minimal Surgical OR Procedures, Minimally Invasive Surgical OR Minimal Access Surgical Procedures OR Minimal Surgical Procedures OR Procedure, Minimal Surgical OR Minimally Invasive Surgical Procedures OR Minimal Surgical Procedure OR Surgical Procedure, Minimal OR Surgical Procedures, Minimal OR Surgical Procedures, Minimal Access OR minimally invasive surgical procedure OR minimal access surgical procedure OR laparoscopy OR laparoscopies OR laparoscope OR laparoscopes OR laparos* OR laparoscopic OR Celioscopy OR Celioscopies OR Peritoneoscopy OR Peritoneoscopies OR Surgical Procedures, Laparoscopic OR Procedures, Laparoscopic Surgical OR Surgery, Laparoscopic OR Laparoscopic Surgical Procedure OR Procedure, Laparoscopic Surgical OR Laparoscopic Surgical Procedures OR Laparoscopic Surgery OR Laparoscopic Surgeries OR Surgeries, Laparoscopic OR Surgical Procedure, Laparoscopic OR thoracoscopy OR thoracoscopies OR thoracoscope OR thoracoscopes OR thoracos* OR thoracoscopic Endoscopy, Pleural OR Endoscopies, Pleural OR Pleural Endoscopies OR Pleural Endoscopy OR Pleuroscopy OR Pleuroscopies OR Surgical Procedures, Thoracoscopic OR Surgical Procedure, Thoracoscopic OR Thoracoscopic Surgical Procedure OR Surgery, Thoracoscopic OR Surgeries, Thoracoscopic OR Thoracoscopic Surgeries OR Thoracoscopic Surgery OR Thoracoscopic Surgical Procedures OR VATS OR VATSS OR Surgeries, Video‐Assisted Thoracic OR Surgery, Video‐Assisted Thoracic OR Thoracic Surgeries, Video‐Assisted OR Thoracic Surgery, Video Assisted OR Video‐Assisted Thoracic Surgeries OR Surgery, Thoracic, Video‐Assisted OR Video‐Assisted Thoracic Surgery OR Video Assisted Thoracic Surgery OR Video‐Assisted Thoracoscopic Surgery OR Surgeries, Video‐Assisted Thoracoscopic OR Surgery, Video‐Assisted Thoracoscopic OR Thoracoscopic Surgeries, Video‐Assisted OR Thoracoscopic Surgery, Video‐Assisted OR Video Assisted Thoracoscopic Surgery OR Video‐Assisted Thoracoscopic Surgeries OR videolaparoscopy OR videolaparoscopies)
2. For childhood cancer the following MeSH headings and text words were used:
(((lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR sarcoma, Ewing's OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom*) OR (pediatric oncology OR paediatric oncology)) OR (childhood cancer OR childhood tumor OR childhood tumors)) OR (brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm*)
3. For children the following MeSH headings and text words were used:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
4. ForCochrane RCTs/CCTs the following MeSH headings and text words will be used:
((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) AND (humans[mh])
Final search 1 AND 2 AND 3 AND 4
[pt = publication type; tiab = title, abstract; sh = subject heading; mh = MeSH term; *=one or more characters;RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 3. Search strategy for Embase (OVID)
1. For thedifferent surgical interventions the following Emtree terms and text words were used:
1. (MIS or minimally invasive surgical procedures or minimally invasive surgery or minimal surgical procedure or minimal access surgical procedures or minimal surgical procedures or minimally invasive surgical procedure or minimal access surgical procedure or minimally invasive procedure or minimally invasive procedures).mp. 2. (laparoscopy or laparoscopies or celioscopy or celioscopies or peritoneoscopy or peritoneoscopies or laparoscopic surgical procedure or laparoscopic surgical procedures or laparoscopic surgery or laparoscopic surgeries).mp. 3. (laparoscope or laparoscopes or laparos$ or laparoscopic).mp. 4. (thoracoscopy or thoracoscopies or pleural thoracoscopic endoscopy or pleural thoracoscopic endoscopies or pleural endoscopy or pleural endoscopies or pleuroscopy or pleuroscopies or thoracoscopic surgical procedure or thoracoscopic surgical procedures or thoracoscopic surgery or thoracoscopic surgeries).mp. 5. (thoracoscope or thoracoscopes or thoracos$ or thoracoscopic).mp. 6. (VATS or VATSS or videolaparoscopy or videolaparoscopies or (video adj assisted thoracoscopic surgery) or (video adj assisted thoracoscopic surgeries) or (video adj assisted thoracic surgery)).mp. 7. minimally invasive surgery/ or laparoscopy/ or laparoscope/ or thoracoscopy/ or thoracoscope/ or abdominal surgery/ or thorax surgery/ or laparoscopic surgery/ or endoscopic surgery/ 8. or/1‐7
2. For childhood cancer the following Emtree terms and text words were used:
1. (lymphoma or lymphom$ or hodgkin or hodgkin$ or T‐cell or B‐cell or non‐hodgkin).mp. 2. (sarcoma or sarcom$ or Ewing$ or osteosarcoma or osteosarcom$ or wilms tumor or wilms$).mp. 3. (nephroblastom$ or neuroblastoma or neuroblastom$ or rhabdomyosarcoma or rhabdomyosarcom$ or teratoma or teratom$ or hepatoma or hepatom$ or hepatoblastoma or hepatoblastom$).mp. 4. (PNET or medulloblastoma or medulloblastom$ or PNET$ or neuroectodermal tumors or primitive neuroectodermal tumor$ or retinoblastoma or retinoblastom$ or meningioma or meningiom$ or glioma or gliom$).mp. 5. (pediatric oncology or paediatric oncology).mp. 6. ((childhood adj cancer) or (childhood adj tumor) or (childhood adj tumors) or childhood malignancy or (childhood adj malignancies) or childhood neoplasm$).mp. 7. ((pediatric adj malignancy) or (pediatric adj malignancies) or (paediatric adj malignancy) or (paediatric adj malignancies)).mp. 8. ((brain adj tumor$) or (brain adj tumour$) or (brain adj neoplasms) or (brain adj cancer$) or brain neoplasm$).mp. 9. (central nervous system tumor$ or central nervous system neoplasm or central nervous system neoplasms or central nervous system tumour$).mp. 10. intracranial neoplasm$.mp. 11. LYMPHOMA/ or brain tumor/ or central nervous system tumor/ or teratoma/ or sarcoma/ or osteosarcoma/ 12. nephroblastoma/ or neuroblastoma/ or rhabdomyosarcoma/ or hepatoblastoma/ or medulloblastoma/ or neuroectodermal tumor/ or retinoblastoma/ or meningioma/ or glioma/ or childhood cancer/ 13. or/1‐12
3. Forchildren the following Emtree terms and text words were used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
4. For Cochrane RCTs/CCTs the following Emtree terms and text words were used:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. Human/ 11. 9 and 10
Final search 1 and 2 and 3 and 4
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; sh = subject heading; ti,ab = title, abstract; / = Emtree term; $=zero or more characters ; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 4. Search strategy for conference proceedings
The SIOP and ASCO abstracts (2011 to 2013) were searched with the following search terms:
Minimal invasive; Minimally invasive; Surgery; Open surgery; Laparoscopy; Laparoscopic; Laparotomy; Thoracoscopy; Thoracotomy; Abdominal; Thoracic; Resection
Appendix 5. Search strategy for ongoing trial registers
1. http://www.controlled‐trials.com (ISRCTN register, NIH register)
Search terms were: Minimal invasive; Minimally invasive; Surgery; Open surgery; Laparoscopy; Laparoscopic; Laparotomy; Thoracoscopy; Thoracotomy; Abdominal; Thoracic; Resection
2. http://clinicaltrials.gov (NIH register) was searched via advanced search page, and the box Additional Criteria, Age Group: Child (birth‐17) was ticked off.
Search terms were: Minimal invasive; Minimally invasive; Surgery; Open surgery; Laparoscopy; Laparoscopic; Laparotomy; Thoracoscopy; Thoracotomy; Abdominal; Thoracic; Resection
3. http://apps.who.int/trialsearch (WHO ICTRP register) was searched via the advanced search page. The button *search for clinical trials in children" was used.
Search terms were: Minimal invasive; Minimally invasive; Surgery; Open surgery; Laparoscopy; Laparoscopic; Laparotomy; Thoracoscopy; Thoracotomy; Abdominal; Thoracic; Resection
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Duarte 2006 | Not a RCT/CCT; case series |
| Ehrlich 2002 | Not a RCT/CCT; questionnaire |
| Iwanaka 2004 | Not a RCT/CCT; retrospective cohort |
| Kelleher 2013 | Not a RCT/CCT; retrospective study |
| Malek 2010 | Not a RCT/CCT; retrospective chart review |
| Shanberg 2006 | Not a RCT/CCT; letter to the editor regarding case reports |
| Stanford 2002 | Not a RCT/CCT; retrospective cohort study |
| Wu 2013 | Not a RCT/CCT; retrospective study; adult patients |
RCT: randomised controlled trial; CCT: controlled clinical trial
Contributions of authors
Elvira van Dalen designed the study, developed the search strategy, identified studies meeting the inclusion criteria, searched for unpublished and ongoing studies, interpreted the results, and wrote and revised the manuscript.
Manou de Lijster designed the study and wrote the protocol, identified studies meeting the inclusion criteria, interpreted the results, and wrote the manuscript and revised the manuscript.
Lieve Leijssen searched for unpublished and ongoing studies during the latest update and critically reviewed the manuscript.
Erna Michiels designed the study, identified studies meeting the inclusion criteria, interpreted the results and critically reviewed the manuscript.
Huib Caron designed the study, interpreted the results and critically reviewed the manuscript.
Leontien Kremer designed the study, identified studies meeting the inclusion criteria, interpreted the results and critically reviewed the manuscript.
Daniel Aronson designed the study, identified studies meeting the inclusion criteria, searched for unpublished studies, interpreted the results and critically reviewed the manuscript.
All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Stichting Kinderen Kankervrij (KIKA), Netherlands.
Declarations of interest
None known.
Joint first authorship with Elvira C van Dalen
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Duarte 2006 {published data only}
- Duarte RJ, Dénes FT, Cristofani LM, Odone‐Filho V, Srougi M. Further experience with laparoscopic nephrectomy for Wilms' tumour after chemotherapy. BJU International 2006;98(1):155‐9. [DOI] [PubMed] [Google Scholar]
Ehrlich 2002 {published data only}
- Ehrlich PF, Newman KD, Haase GM, Lobe TE, Wiener ES, Holcomb GW. Lessons learned from a multi‐institutional randomized controlled study. Journal of Pediatric Surgery 2002;37(3):431‐6. [DOI] [PubMed] [Google Scholar]
Iwanaka 2004 {published data only}
- Iwanaka T, Arai M, Kawashima H, Kudou S, Fujishiro J, Imaizumi S, et al. Endosurgical procedures for pediatric solid tumors. Pediatric Surgery International 2004;20(1):39‐42. [DOI] [PubMed] [Google Scholar]
Kelleher 2013 {published data only}
- Kelleher CM, Smithson L, Nguyen LL, Casadiego G, Nasr A, Irwin MS, et al. Clinical outcomes in children with adrenal neuroblastoma undergoing open versus laparoscopic adrenalectomy. Journal of Pediatric Surgery 2013;48(8):1727‐32. [DOI] [PubMed] [Google Scholar]
Malek 2010 {published data only}
- Malek MM, Mollen KP, Kane TD, Shah SR, Irwin C. Thoracic neuroblastoma: a retrospective review of our institutional experience with comparison of the thoracoscopic and open approaches to resection. Journal of Pediatric Surgery 2010;45(8):1622‐6. [DOI] [PubMed] [Google Scholar]
Shanberg 2006 {published data only}
- Shanberg AM, Perer E, Matsunaga G. Re: Laparoscopic nephrectomy for Wilms tumor after chemotherapy: initial experience. Journal of Urology 2006;175(2):788. [DOI] [PubMed] [Google Scholar]
Stanford 2002 {published data only}
- Stanford A, Upperman JS, Nguyen N, Barksdale E Jr, Wiener ES. Surgical management of open versus laparoscopic adrenalectomy: outcome analysis. Journal of Pediatric Surgery 2002;37(7):1027‐9. [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu Z, Zhou J, Wang X, Li YB, Niu T, Peng B. Laparoscopic splenectomy for treatment of splenic marginal zone lymphoma. World Journal of Gastroenterology 2013;19(24):3854‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Al‐Shanafey 2008
- Al‐Shanafey S, Habib Z. Feasibility and safety of laparoscopic adrenalectomy in children: special emphasis on neoplastic lesions. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2008;18(2):306‐9. [DOI] [PubMed] [Google Scholar]
Bax 2005
- Bax NM. Laparoscopic surgery in infants and children. European Journal of Pediatric Surgery 2005;15(5):319‐24. [DOI] [PubMed] [Google Scholar]
Berends 1994
- Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994;344(8914):58. [DOI] [PubMed] [Google Scholar]
Buunen 2009
- Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long‐term outcome of a randomised clinical trial. Lancet Oncology 2009;10(1):44‐52. [DOI] [PubMed] [Google Scholar]
Castilho 2002
- Castilho LN, Castillo OA, Dénes FT, Mitre AI, Arap S. Laparoscopic adrenal surgery in children. Journal of Urology 2002;168(1):221‐4. [PubMed] [Google Scholar]
Duarte 2009
- Duarte RJ, Denez FT, Cristofani LM, Srougi M. Laparoscopic nephrectomy for Wilms' tumor. Expert Review of Anticancer Therapy 2009;9(6):753‐61. [DOI] [PubMed] [Google Scholar]
Georgeson 2000
- Georgeson KE, Owings E. Advances in minimally invasive surgery in children. American Journal of Surgery 2000;180(5):362‐4. [DOI] [PubMed] [Google Scholar]
Georgeson 2003
- Georgeson K. Minimally invasive surgery in neonates. Seminars in Neonatology 2003;8(3):243‐8. [DOI] [PubMed] [Google Scholar]
Johnson 1997
- Johnson A. Laparoscopic surgery. Lancet 1997;349(9052):631‐5. [DOI] [PubMed] [Google Scholar]
Lacy 2002
- Lacy AM, García‐Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy‐assisted colectomy versus open colectomy for treatment of nonmetastatic colon cancer: a randomized trial. Lancet 2002;359(9325):2224‐9. [DOI] [PubMed] [Google Scholar]
Leclair 2007
- Leclair MD, Sarnacki S, Varlet F, Heloury Y. Minimally‐invasive surgery in cancer children [Vidéochirurgie et cancer de l'enfant]. Bulletin du Cancer 2007;94(12):1087‐90. [DOI] [PubMed] [Google Scholar]
Leung 2004
- Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomized trial. Lancet 2004;363(9416):1187‐92. [DOI] [PubMed] [Google Scholar]
Liang 2008
- Liang Y, Li G, Chen P, Yu J. Laparoscopic versus open colorectal resection for cancer: a meta‐analysis of results of randomized controlled trials on recurrence. European Journal of Surgical Oncology 2008;34(11):1217‐24. [DOI] [PubMed] [Google Scholar]
Metzelder 2007
- Metzelder ML, Kuebler JF, Shimotakahara A, Glueer S, Grigull L, Ure BM. Role of diagnostic and ablative minimally invasive surgery for pediatric malignancies. Cancer 2007;109(11):2343‐8. [DOI] [PubMed] [Google Scholar]
Milsom 1998
- Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. Journal of the American College of Surgeons 1998;187(1):46‐54. [DOI] [PubMed] [Google Scholar]
Saenz 1997
- Saenz NC, Conlon KC, Aronson DC, LaQuaglia MP. The application of minimal access procedures in infants, children, and young adults with pediatric malignancies. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 1997;7(5):289‐94. [DOI] [PubMed] [Google Scholar]
Sailhamer 2003
- Sailhamer E, Jackson CC, Vogel AM, Kang S, Wu Y, Chwals WJ, et al. Minimally invasive surgery for pediatric solid neoplasms. American Surgeon 2003;69(7):566‐8. [PubMed] [Google Scholar]
Schmidt 2007
- Schmidt AI, Engelmann C, Till H, Kellnar S, Ure BM. Minimally‐invasive pediatric surgery in 2004: a survey including 50 German institutions. Journal of Pediatric Surgery 2007;42(9):1491‐4. [DOI] [PubMed] [Google Scholar]
Song 2009
- Song SY, Na KJ, Oh SG, Ahn BH. Learning curves of minimally invasive esophageal cancer surgery. European Journal of Cardio‐Thoracic Surgery 2009;35(4):689‐93. [DOI] [PubMed] [Google Scholar]
Spurbeck 2004
- Spurbeck WW, Davidoff AM, Lobe TE, Rao BN, Schropp KP, Shochat SJ. Minimally invasive surgery in pediatric cancer patients. Annals of Surgical Oncology 2004;11(3):340‐3. [DOI] [PubMed] [Google Scholar]
Ure 2000
- Ure BM, Bax NM, Zee DC. Laparoscopy in infants and children: a prospective study on feasibility and the impact on routine surgery. Journal of Pediatric Surgery 2000;35(8):1170‐3. [DOI] [PubMed] [Google Scholar]
Varlet 2009
- Varlet F, Stephan JL, Guye E, Allary R, Berger C, Lopez M. Laparoscopic radical nephrectomy for unilateral renal cancer in children. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2009;19(2):148‐52. [DOI] [PubMed] [Google Scholar]
Warmann 2003
- Warmann S, Fuchs J, Jesch NK, Schrappe M, Ure BM. A prospective study of minimally invasive techniques in pediatric surgical oncology: preliminary report. Medical and Pediatric Oncology 2003;40(3):155‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
De Lijster 2010
- Lijster MS, Bergevoet RM, Dalen EC, Michiels EMC, Caron HN, Kremer LCM, et al. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008403] [DOI] [PubMed] [Google Scholar]
De Lijster 2012
- Lijster MS, Bergevoet RM, Dalen EC, Michiels EMC, Caron HN, Kremer LCM, et al. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD008403.pub2] [DOI] [PubMed] [Google Scholar]


