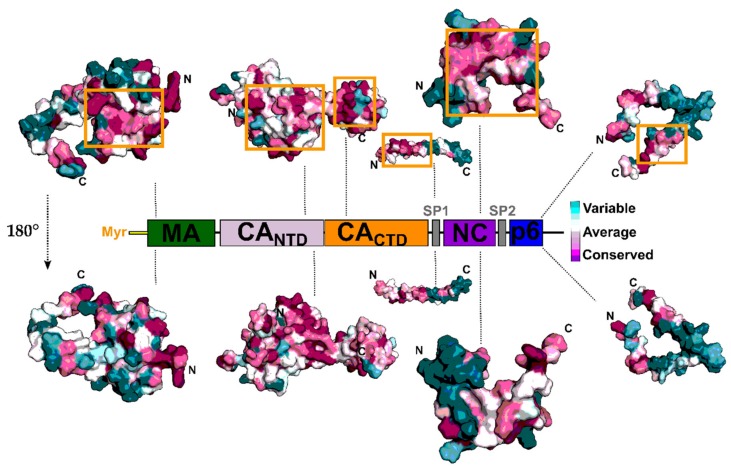
Figure 2.
Surface conservation of the HIV-1 Gag polyprotein and inhibitor target sites. Alignment of 9547 HIV-1 Gag sequences were retrieved from the HIV Los Alamos database (www.hiv.lanl.gov). Sequences were aligned against the HxBc2 reference. Conservation analysis was performed using the ConSurf server [10]. Structures of the HIV-1 Gag domains with PDB entries: matrix, 2H3Z; capsid, 6ES8; sp2, 1U57; nucleocapsid, 2M3Z; p6, 2C55. Orange boxes represent target sites for inhibitor binding highlighted in this review. Low conservation in light cyan to high conservation in dark purple.Gag’s constituent proteins act at different points in the viral life cycle. MA binds specifically to phosphoinositide 4,5-bisphosphate (PI [4,5]P2) and specific phospholipids on the plasma membrane, triggering the exposure of an attached myristoyl (myr) chain and directing Gag to the membrane. This membrane interaction is required for the correct incorporation of the viral envelope protein (Env) into the budding virus [11,12,13,14]. In the late stages of the replication cycle, CA is responsible for the assembly of Gag at the plasma membrane by providing intermolecular contact sites for Gag oligomerization at the plasma membrane [15,16]. In the early stages of replication, CA disassembly regulates the process of reverse transcription, and its engagement of cellular transportins and nuclear pore components facilitate the import of the viral pre-integration complex into the nucleus, where integration takes place [17]. NC functions as a nucleic acid chaperone at multiple steps in the HIV-1 replication cycle, and it’s overall positively charged character and two zinc-finger motifs allow it to interact with viral genomic RNA via the RNA packaging signal and thereby facilitate virion assembly [18,19,20,21]. Finally, the p6 domain (late domain) recruits the endosomal sorting complex required for transport (ESCRT) machinery to promote virus budding and final release [22]. Two spacer peptides (SP1 and SP2) flanking the NC domain regulate the kinetics of Gag maturation, and SP1 also provides, as part of the C-terminus of CA, another Gag-Gag multimerization interface [23,24]. Because Gag functions in so many different aspects of viral infection and replication, Gag inhibitors have the potential to exert their effects in both early and late stages of the replication cycle, making this polyprotein a particularly attractive target for the development of new therapeutics.