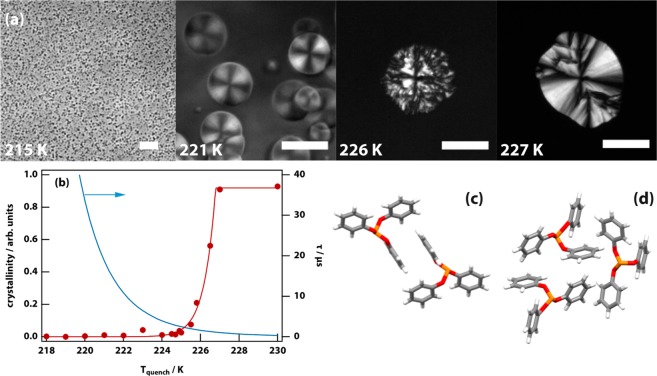
Figure 1.
Microscopy of the liquid–liquid transition (LLT) in triphenyl phosphite (TPP) was used to determine the degree of crystallinity and to discover a new crystal polymorph. (a) Microscopy images of the formation of the liquid 2 phase of TPP at different quench temperatures. The phase-contrast image taken at 215 K shows high probability nucleation. The remaining polarization-microscopy images show the transition proceeding by nucleation and growth. A faint Maltese cross visible at 221 K indicates weak long-range ordering. The scale bar is 50 μm. (b) Liquid 2 droplet crystallinity as a function of quench temperature (red disks, the red line is a guide to the eye). The brightness of the droplet relative to the surrounding untransformed liquid under crossed polarizers is a proxy for crystallinity. Above ∼225.5 K the crystallinity shoots up, indicating that the droplet is composed largely or entirely of crystalline TPP. Droplet size varies inversely with quench temperature, and droplets <217 K are too small to be measured reliably; see (a). Also shown is a Vogel–Fulcher–Tammann fit to the experimentally determined relaxation time in liquid 1 (blue line).25 (c) The molecular packing in the new crystal polymorph (crystal 1) exhibits parallel π-stacking of the phenoxy rings. (d) In contrast, the packing in the thermodynamically most stable crystal polymorph (crystal 2) exhibits T-shaped π-stacking.