Figure 2. Characterization of the cluster variants.
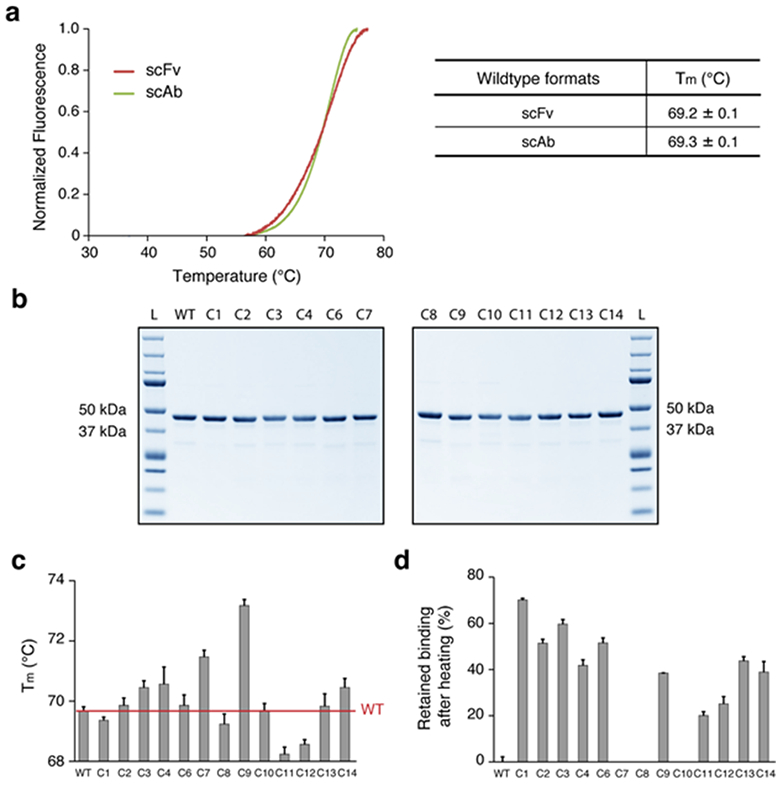
(a) Melting temperatures (Tm) of the anti-HA33 antibody in scFv and scAb formats. scFv were generated using the approach summarized in Supplementary Figure 2. (b) SDS-PAGE of the HA33 and the 13 variants expressed in scAb format (expected size: ~43 kDa). L, protein ladder. (c) Tm measurements of the designed variants expressed as scAbs. (d) Retained binding activity of the designed scAb variants after thermal challenge at 70 °C for 1 hour. The percent binding affinity retained for each variant was calculated by setting the half maximal effective concentration (EC50) of each variant without the heat treatment as 100% and the EC50 of the wildtype after the thermal challenge as 0% (the C7, C8, and C10 variants were worse than the wildtype and set to 0). For (a), (c) and (d), averages are calculated as mean with error indicating s.d., repeated in triplicates.
