Figure 1.
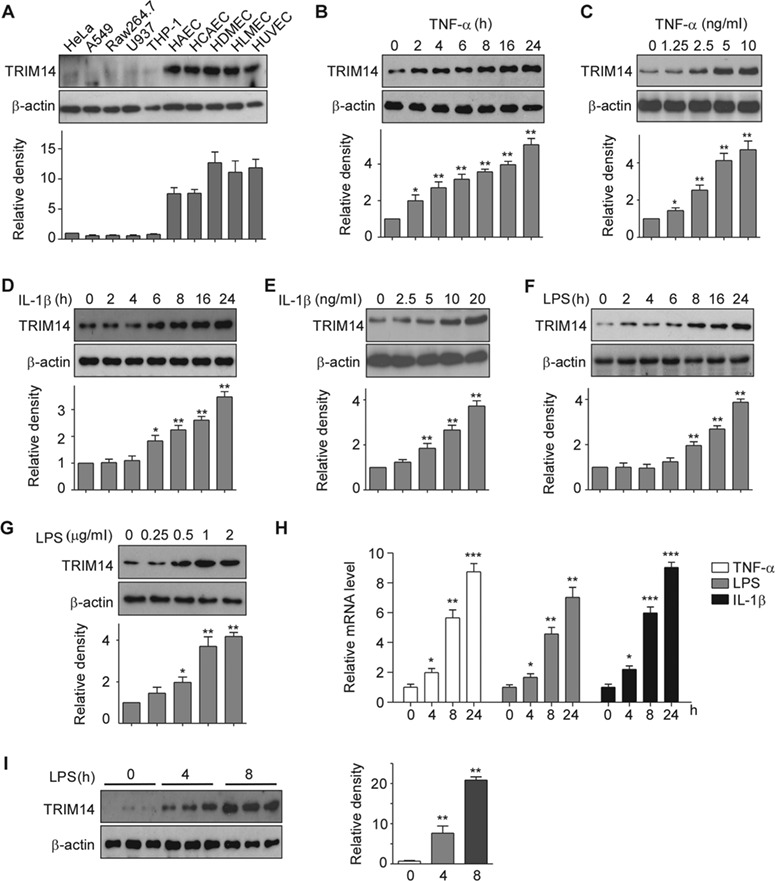
Expression of TRIM14 in human ECs. (A) Whole-cell lysates were isolated from human primary vascular ECs including HAEC, HCAEC, HDMEC, HLMEC, HUVEC, and other human cell lines as indicated and used for western blot assays to detect TRIM14. β-actin served as a loading control. Relative fold changes were determined by densitometry and normalized to β-actin. (B–G) HUVECs were treated with 10 ng/ml TNF-α (B), 10 ng/ml IL-1β (D), or 1 μg/ml LPS (F) for 0, 2, 4, 6, 8, 16, and 24 h, respectively. HUVECs were also treated with TNF-α (C), IL-1β (E), or LPS (G) in different doses as indicated for 24 h. Whole-cell lysates were harvested for detection of TRIM14 protein levels, β-actin served as a loading control. Western blot bands were quantified using Gel-Pro Analyzer software and presented as fold changes under the images. *P < 0.05, **P < 0.01 vs. 0 group by Student’s t-test. (H) HUVECs were stimulated with TNF-α, IL-1β, and LPS for 24 h. Total RNA was extracted, and mRNA levels of TRIM14 were detected by qPCR. Data are presented as mean ± SD (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS-treated group. (I) TRIM14 protein levels in the aortas of adult C57BL/6 mice treated with LPS for different times as indicated. Fold-change of the protein levels were determined by densitometry and normalized to β-actin. Quantitative data are presented as mean ± SD (n = 3); **P < 0.01 vs. 0 h group by Student’s t-test.
