Figure 5.
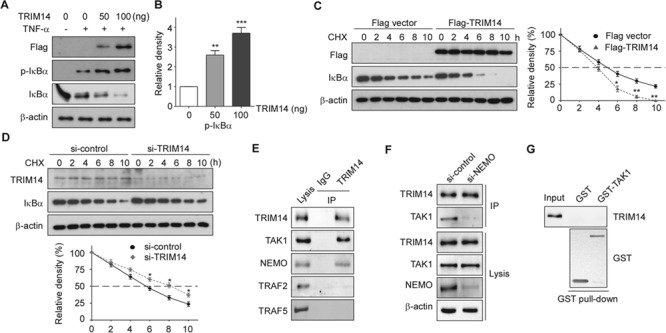
TRIM14 facilitated IκBα phosphorylation and degradation in activated ECs. (A) HUVECs were transiently transfected with 50 ng or 100 ng Flag-TRIM14 or empty vector for 24 h and then treated with TNF-α for 15 min. Cell lysates were collected, and western blots were performed to detect phosphorylation of IκBα and total protein levels of IκBα. (B) Relative fold changes of phosphorylated protein was determined by densitometry and normalized to β-actin. Data are presented as mean ± SD (n = 3); **P < 0.01, ***P < 0.001. (C) Flag-TRIM14 or empty vector transfected HUVECs were incubated with 50 μg/ml CHX for indicated time intervals. IκBα were detected with IκBα antibody by western blot. Quantitative analysis of IκBα protein levels was shown. Data are presented as mean ± SD (n = 3); *P < 0.05, **P < 0.01. (D) HUVECs were transfected with control siRNAs or TRIM14 siRNAs for 24 h and then treated with 50 μg/ml CHX for indicated time intervals. Cell lysates were collected for western blot. Quantitative analysis of IκBα protein levels was shown. Data are presented as mean ± SD (n = 3); *P < 0.05. (E) HUVECs were treated with TNF-α for 15 min, and whole-cell lysates were immunoprecipitated with TRIM14 antibody (mouse) or mouse IgG as control. The precipitates were immunoblotted with the indicated antibodies. (F) HUVECs were transiently transfected with control siRNAs or NEMO siRNAs for 24 h and then treated with TNF-α for 15 min, and whole-cell lysates were immunoprecipitated with TRIM14 antibody (mouse) or mouse IgG as control. The precipitates were immunoblotted with TAK1 antibody. (G) Purified GST or GST-TAK1 protein were incubated with the cell lysates of TNF-α treated HUVECs. After GST pull-down experiment, the elute protein and cell lysates were analyzed by western blot with indicated antibodies.
