Figure 8.
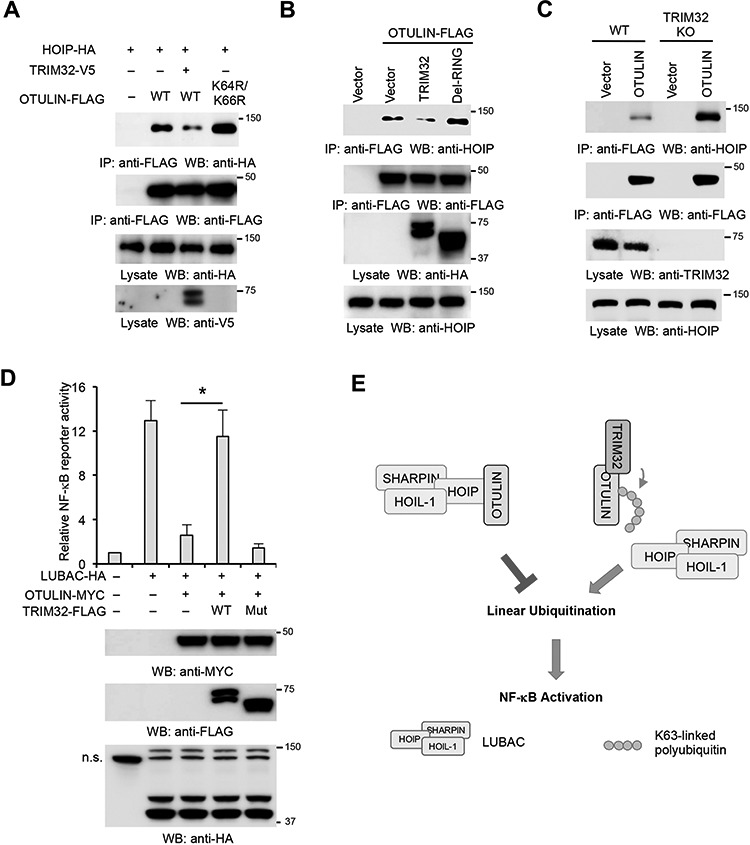
The non-proteolytic ubiquitination perturbs OTULIN–HOIP interaction. (A) HOIP, TRIM32, and OTULIN or the K64R/K66R mutant were transfected into HEK293 cells in the indicated combination. After 48 h, cell lysates were immunoprecipitated and blotted with the indicated antibodies. (B) OTULIN-FLAG was transfected with TRIM32 or the RING deletion mutant (Mut) into HEK293 cells. After 48 h, cell lysates were immunoprecipitated with anti-FLAG antibody and blotted as indicated. (C) OTULIN-FLAG was transfected into HEK293 and the TRIM32 knockout cells. After 48 h, cell lysates were immunoprecipitated with anti-FLAG antibody and blotted as indicated. (D) TRIM32 or the RING deletion mutant was transfected into HEK293 cells along with OTULIN, LUBAC, NF-κB luciferase reporter, and pRL-SV40 in the indicated combination. After 48 h, luciferase activities were measured. *P < 0.05. The protein expression levels of TRIM32, LUBAC, and OTULIN were determined by western blotting. The non-specific band (n.s.) is indicated. (E) The working model for TRIM32-mediated OTULIN ubiquitination and NF-κB activation.
