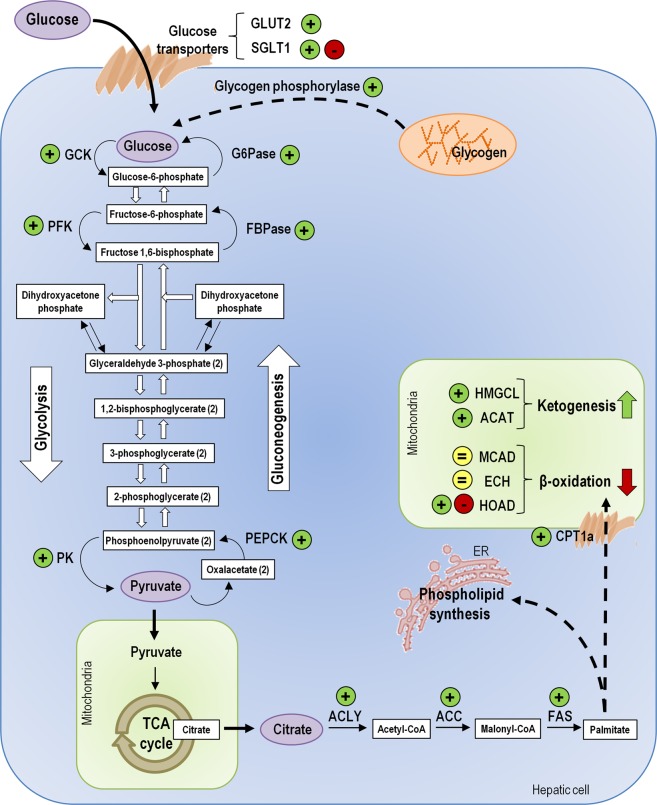
Figure 9.
Schematic representation of the metabolic pathways proposed to be modulated by FGF21 in the zebrafish hepatic cells. Diagram shows the main pathways studied, and the transporters and enzymes whose mRNA expression (and activity for some of them) was shown to be upregulated (+), downregulated (−) or unaltered (=) by FGF21 treatment in vivo and/or in vitro. Based on the results of the present study, we hypothesize that FGF21 leads to an increase in the amount of glucose in the hepatic cells by enhancing its entrance into the cells, gluconeogenesis and glycogen degradation. Interestingly, we also observed evidence in favor of FGF21 stimulating not only gluconeogenesis but also glycolysis. We hypothesize that the final product of glycolysis, pyruvate, after being converted into citrate, might be released into the cytoplasm and be used as a substrate for fatty acid biosynthesis. Our results do not support that the increase in fatty acid synthesis is related to its β-oxidation, but instead it might be related to the synthesis of phospholipids for maintaining cell membrane structure. In addition, we propose that FGF21 might stimulate ketogenesis. ACAT, Acetyl-CoA acyltransferase; ACC, Acetyl-CoA carboxylase; ACLY, ATP citrate lyase; CPT1a, Carnitine palmitoyltransferase 1a; ECH, Enoyl-CoA hydratase; ER, endoplasmic reticulum; FAS, Fatty acid synthase; FBPase, Fructose 1,6-bisphosphatase; G6Pase, Glucose 6-phosphatase; GCK, Glucokinase; GLUT2, Glucose transporter 2; HMGCL, 3-hydroxy-3-methylglutaryl-CoA lyase; HOAD, 3-hydroxyacyl-CoA dehydrogenase; MCAD, Medium-chain acyl-coenzyme A dehydrogenase; PEPCK, Phosphoenolpyruvate carboxykinase; PFK, Phosphofructokinase; PK, Pyruvate kinase; SGLT1, sodium-glucose cotransporter 1; TCA cycle, tricarboxylic acid cycle (Krebs cycle).