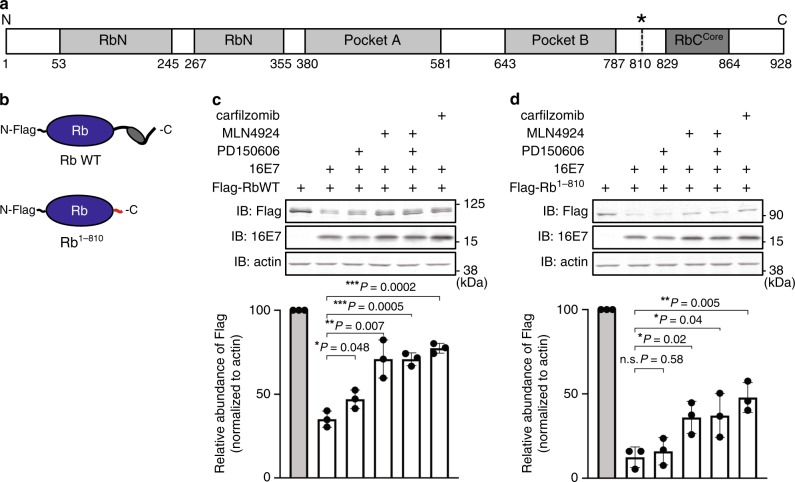
Fig. 1. Contribution of calpain and a cullin E3 ligase to Rb degradation.
a Schematic representation of Rb’s domain structure. Rb contains two structured domains (RbN and Pocket A/B, gray), which are flanked by disordered regions including RbCCore (dark gray) in the C-terminal region. The asterisk denotes the calpain cleavage residue Lys 810. b Schematic view of N-terminally Flag-tagged full-length Rb protein (Rb WT) and the cleavage product (Rb1–810). The RbCCore and cleavage-exposed tail are highlighted in dark gray and red, respectively. c, d Representative images of immunoblots of Rb mutants in the presence of 16E7 and inhibitors. HEK293T cells were transfected with Rb WT (c) and Rb1–810 (d), as well as 16E7 or empty vector as indicated, for 24 h. Cells were treated with vehicle (DMSO), 100 μM PD150606, 1 μM MLN4924, and/or 1 μM carfilzomib for the last 4 h, and the lysates were subjected to immunoblotting with the indicated antibodies. The graph plots the relative band intensities as means ± the standard deviations (SD) from three repeat experiments. ns not significant. *P < 0.05; **P < 0.01; ***P < 0.001 (two-tailed unpaired t-test). Source data are provided as a Source Data file.