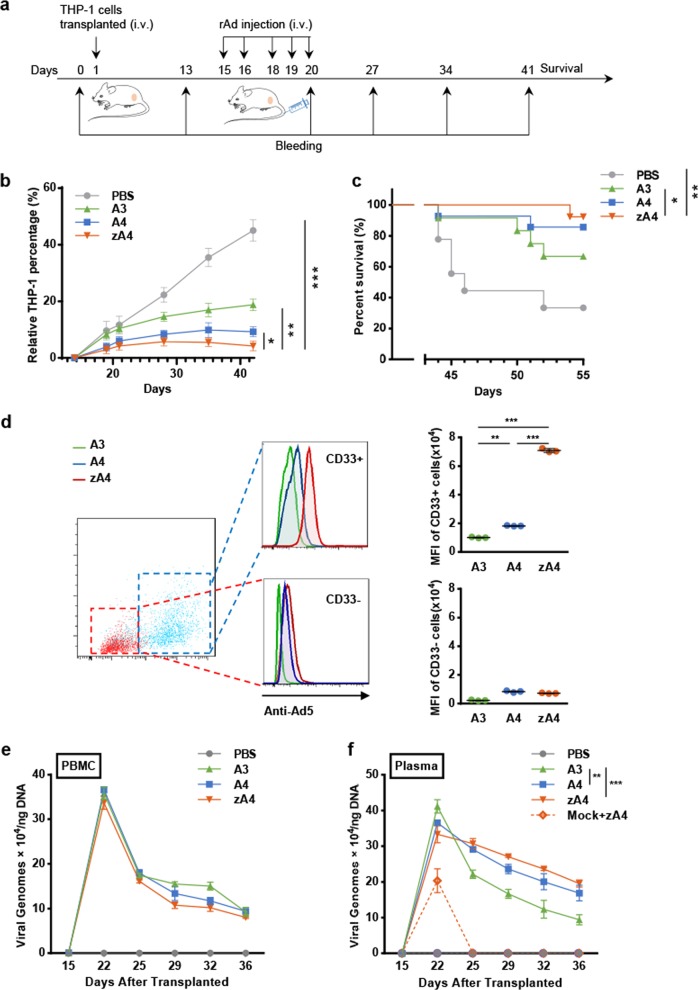
Fig. 6.
Targeting ability and anti-cancer effects of recombinant Ad vectors in the subcutaneous model. a Treatment strategy used in the subcutaneous model. Freshly cultured THP-1 cells (1 × 107) were injected subcutaneously into the right flank of mice. Recombinant Ad (2 × 108 IU) was administered intravenously each time. Each mouse was administered 1 × 109 IU of recombinant Ad during the entire treatment. The mice were killed 35 days after tumor cell transplantation. b Tumor burden curve as indicated by the relative percentage of THP-1 cells. The proportion of THP-1 cells in the PBMCs of each mouse was detected using an anti-CD33 antibody by flow cytometry. c Kaplan–Meier analysis of animal end point survival after intravenous treatment with A3, A4, and zA4. d The targeting ability of recombinant Ad in PBMCs of tumor-bearing mice was detected by flow cytometry. The PBMCs from mice treated with A3, A4, and zA4 were gated according to staining with CD45 and CD33 antibodies. The targeting ability of the recombinant Ad was represented by RFP expression by adenoviruses. e Detection of viral load in PBMCs. The viral genome was detected by quantitative real-time PCR. f Evaluation of the viral genome in plasma. The quantity of the viral load was detected by quantitative real-time PCR. “Mock+zA4” indicates the viral load of nontumor-bearing mice with intravenous injection of zA4. Error bars represent the SEM. *P < 0.05; **P < 0.01; ***P < 0.001