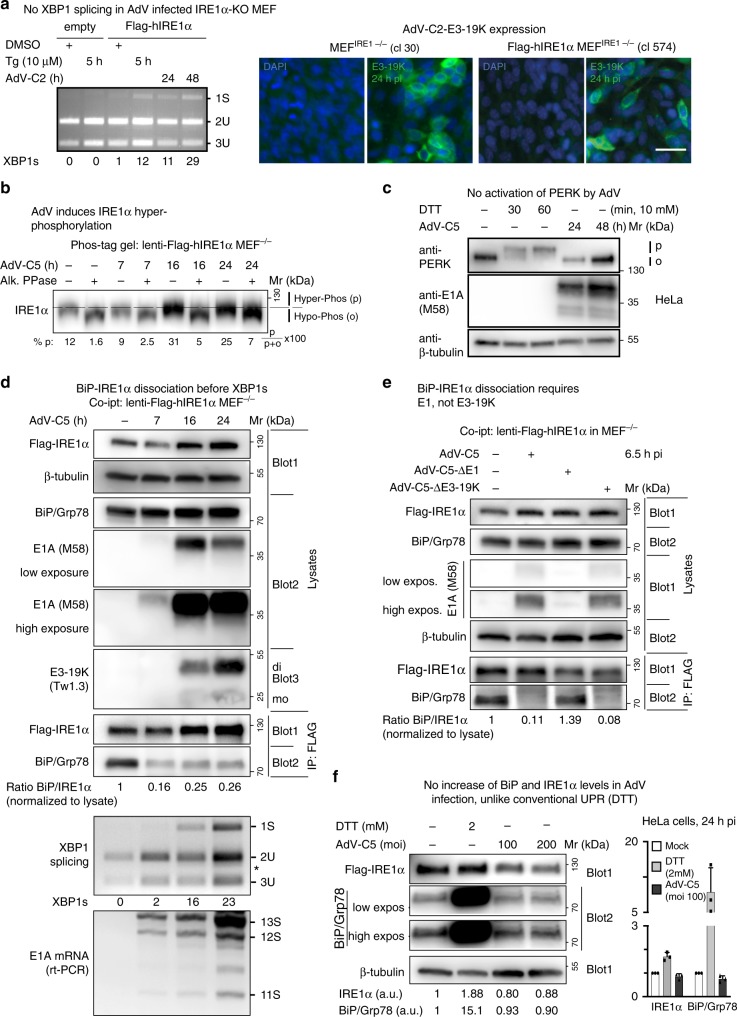
Fig. 2. AdV infection of mouse embryonic fibroblasts induces XBP1 splicing and displaces BiP/Grp78 from IRE1α.
a Flag-IRE1α-expressing MEFs but not IRE1α-KO MEFs induce XBP1s upon AdV-C2 infection (MOI 300) or thapsigargin (Tg, 10 µM) treatment (left panel). Representative immunofluorescence images with the anti-19K antibody 3A9 showing 19K expression in AdV-C-infected IRE1α-KO MEFs and Flag-IRE1α-expressing IRE1α-KO MEFs 24 hpi (clones 30 and 574, respectively, right panel, scale bar, 20 µm). Three independent experiments gave similar results. Source data are provided as a Source Data file. b AdV induces phosphorylation of IRE1α. Flag-IRE1α expressing IRE1α-KO MEFs at 7, 16, and 24 hpi with AdV-C5 (MOI 300) immunoblotted with anti-IRE1α antibody. Lysates were resolved on a 6% SDS-PAGE gel containing 25 µM Phos-tag. Samples were treated with or without alkaline phosphatase. Phosphorylated (p) and hypophosphorylated (o) forms of IRE1α are indicated by the dashed lines, and the percentage of IRE1α phosphorylated was calculated as indicated. Lysates are the same as in panel D demonstrating β-tubulin loading. Three independent experiments gave similar results. Source data are provided as a Source Data file. c AdV-C5 infection of HeLa cells (MOI 200) does not activate PERK, unlike treatment of cells with the reducing agent DTT. Activated phosphorylated PERK is indicated by p, and the inactive form by o. Two independent experiments gave similar results. d BiP displacement from IRE1α occurs before XBP1 splicing in Flag-IRE1α-expressing IRE1α-KO MEFs infected with AdV-C5 (MOI 300). Cells were lysed and BiP–IRE1α complexes immunoprecipitated (IP) with anti-Flag antibody, and a western blot with anti-IRE1α, anti-BiP, and anti-β-tubulin antibodies was performed. A separate non-reducing immunoblot probed with anti-19K Tw1.3 antibodies revealed monomeric and dimer forms of 19K indicated as mo and di, respectively. Input lysates were 1% of the immunoprecipitated samples. The corresponding samples were also analyzed for XBP1 splicing and E1A mRNA levels by rt-PCR (reverse transcription polymerase chain reaction), as indicated. Three independent experiments gave similar results. Source data are provided as a Source Data file. e BiP–IRE1α dissociation requires E1, not 19K. Co-immunoprecipitation of Flag-IRE1α and BiP was performed as described in d with AdV mutants lacking E1 (AdV-C5-∆E1) and 19K (AdV-C5-Δ19K) at MOI 300 each. Two independent experiments gave similar results. Source data are provided as a Source Data file. f AdV-C5 infection does not increase BiP/Grp78 and IRE1α levels given in arbitrary units (a.u.), unlike the canonical UPR triggered by DTT (2 mM). The bar graph shows the normalized levels of IRE1α and BiP from three independent experiments. Data show the means ± SD from three independent experiments. Source data are provided as a Source Data file.