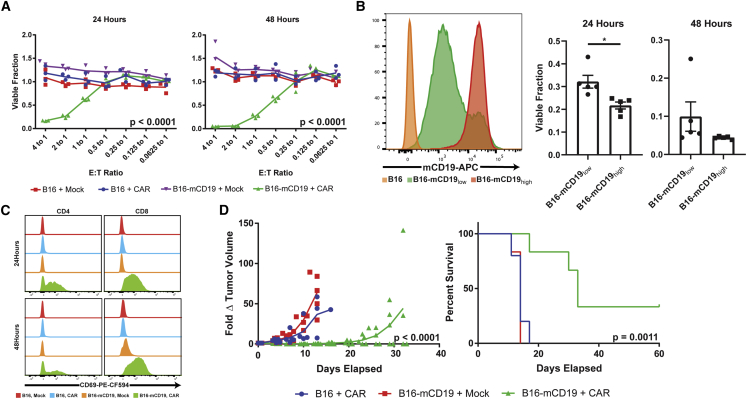
Figure 1.
mCD19 CAR T Cells Exhibit Cytotoxic Activity against a B16-mCD19 Cell Line
(A) Dose- and time-dependent cytotoxicity of mCD19 CAR T cells and mock T cells in in vitro co-cultures against either native B16 cells or a B16 cell line engineered to express mCD19. 24-h E:T = 4, p < 0.0001, F = 49.23, R2 = 0.9486 by ANOVA; 48-h E:T = 4, p < 0.0001, F = 49.65, R2 = 0.9490 by ANOVA. n = 3 independent cultures for each combination, E:T ratio, and time point. (B) Antigen density-dependent mCD19 CAR T cell cytotoxicity against low- and high-mCD19-expressing B16 cell lines at 24 and 48 h of co-culture (n = 5 independent cultures with each cell line, p = 0.0116, t = 3.258, degrees of freedom (df) = 8 by two-tailed unpaired t test). (C) CD69 is upregulated only in antigen-matched co-cultures for both CD4 and CD8 T cells. (D) mCD19 CAR T cells significantly delay B16-mCD19 tumor progression in vivo (left) and confer a survival benefit relative to antigen-mismatched therapy groups. Day 8 tumor volume: p < 0.0001, F = 19.14, R2 = 0.7322 by ANOVA. Kaplan-Meier survival curve: p = 0.0011, df = 2, chi-square = 13.58 by Mantel-Cox test. Number of independent mice in each group is as follows: n = 5 (B16 + CAR), n = 6 (B16-mCD19 + mock), and n = 6 (B16-mCD19 + CAR). Data are shown as mean ± standard error of the mean (SEM). Asterisks indicate statistical significance: ∗p < 0.05.