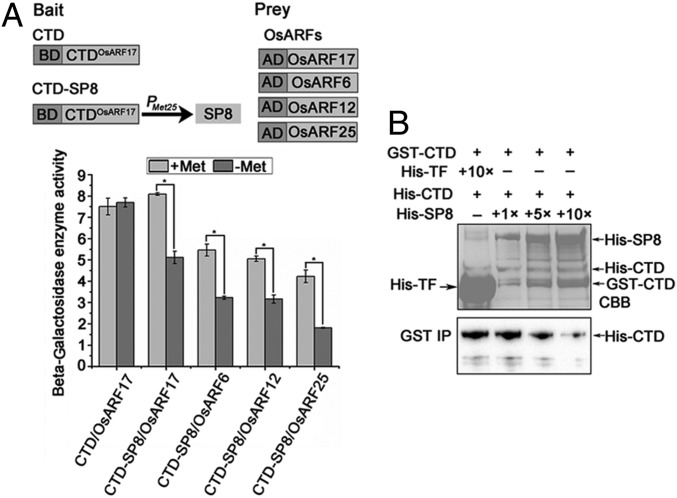
Fig. 2.
SP8 interferes with OsARF17 dimerization. (A) Y3H assays demonstrating the influence of SP8 on OsARF17 dimerization. (Upper) Schematic diagrams of the bait and the prey constructs used in Y3H assays. The CTD domain of OsARF17 ligated into pBridge vector was the bait, and the expression of SP8 was driven by the methionine-inducible Met25 promoter. The OsARFs (OsARF17, OsARF6, OsARF12, OsARF25) were cloned into pGADT7 vectors as prey. (Lower) Yeast cells containing bait and prey vectors were grown on selective media SD/-Trp/-Leu (SD-L-T) for 3 d. The yeast cells were shaken with SD-L-T-H-Ade or SD/-Trp/-Leu/-His/-Met liquid selective media. β-Galactosidase enzyme activity assays with or without SP8. When methionine (20 mM) was present, the expression of SP8 was inhibited. Values shown are the means ± SD of 3 biological replicates. Significant differences were identified using Fisher’s least significant difference tests. *At the top of columns indicates significant difference at P ≤ 0.05. (B) Dose-dependent pull-down assays to assess SP8 interference with the CTD-CTD interaction in vitro. Equal amounts of purified GST–CTD and His–CTD mixed with proteins were incubated with increasing amounts of His–SP8 (0, 2, 10, or 20 μg) or His–TF (negative control) in vitro. The interaction between GST–CTD and His–CTD was weakened by His–SP8. His–CTD proteins were used to pull-down with GST–CTD, and further detected with anti-His antibody. (Upper) The loading of His–SP8, GST–CTD, His–CTD, and His–TF is shown by Coomassie brilliant blue staining.