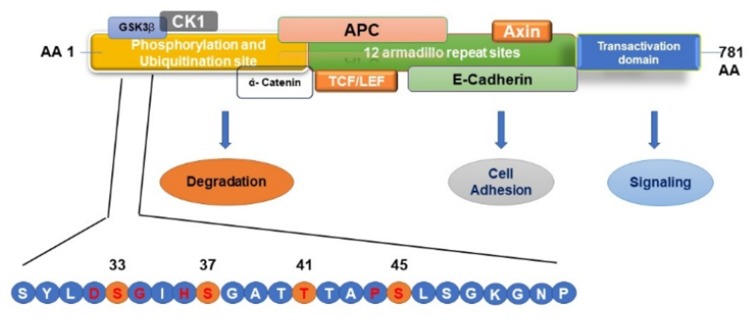
Figure 7.
Schematic Representation of β–catenin phosphorylation, ubiquitination, and protein-binding domains. Mutations in the β–catenin are always found in the degradation-targeting box where CK1 α first phosphorylates Ser45 (shown in yellow circle). This phosphorylation event primes for subsequent phosphorylation by GSK3 β at Ser33, Ser37, and Thr41 (shown in yellow circles). The phosphorylation of these sites initiates the β–catenin degradation by the proteasome system by forming a destruction complex which is formed by axin, adenomatous polyposis coli (APC), and protein phosphatase 2A (PP2A) proteins resulting in the degradation of β-catenin by 26S proteasome system. Mutations in the codons adjacent to the CK1 α and GSK3 β phosphorylation sites (shown in red letter) prevent this destruction complex formation and β-catenin degradation, causing the translocation of β-catenin to the nucleus and activating the transcription of target genes.