Figure 3. The GCP4 crystal structure defines the core structure of all the GCPs.
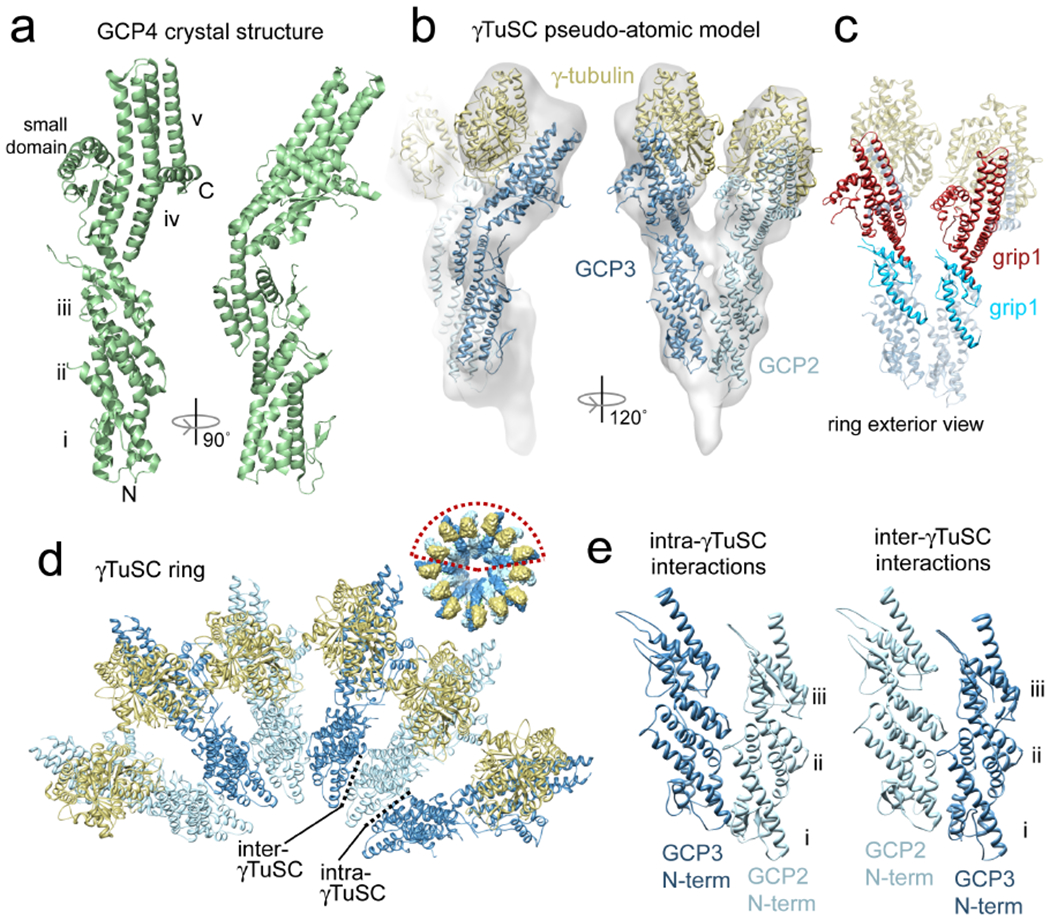
a) The γ-tubulin complex protein 4 (GCP4) crystal structure is shown in two orthogonal views. In the view on the left the five α-helical bundles (i-v), small domain labeled, and N-terminus and C-terminus are labeled. The C-terminal domain, consisting of bundle iv, bundle v and the small domain was shown to directly bind γ-tubulin. b) A pseudo-atomic model of γTuSC. The γ-tubulin crystal structure (gold) and the GCP4 crystal structure (blue) as a stand in for GCP2and GCP3 were fit into the γTuSC cryo-EM reconstruction (semi-transparent surface). The model reveals interaction surfaces between complex components. c) The model also shows the positions of the conserved grip1 and grip2 domains in GCP2 and GCP3 in the context of the full γTuSC. Grip2 is clearly involved in γ-tublin binding. The role of grip1 is more ambiguous; it forms part of the lateral contact surfaces between γTuSCs, as well as part of the faces of GCP2 and GCP3 that are exposed on the outside of the γTuSC ring. d) When the pseudo-atomic model from panel b is fit into the cryo-EM structure of the γTuSC ring (inset), it also reveals the surfaces of GCP2 and GCP3 that are important for oligomerization. γTuSCs interact with each other primarily through the sides of bundles i and ii (Ref. 50). e) The N-terminal domains of GCP2 and GCP3 are shown making intra- and inter-γTuSC contacts, with helical bundles i-iii labeled. Equivalent surfaces of the N-terminal domains of GCP2 and GCP3 are involved in both intra-γTuSC and inter-γTuSC interactions, indicating that a single assembly rule determines the organization of the ring structure. However, the affinities have been modulated such that the stronger intra-γTuSC interactions yield a stable complex, while the weaker inter-γTuSC interactions allow the assembly of γTuSCs into rings to be reversible.
