Box 1. γ-tubulin complex proteins and prior models for their assembly and action.
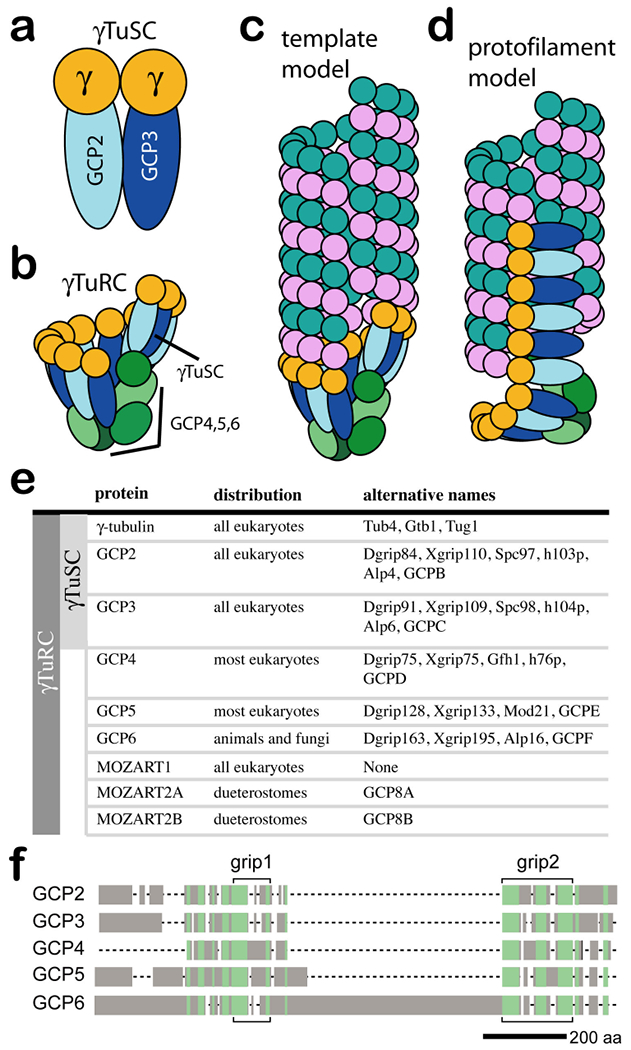
The γ-tubulin small complex (γTuSC) is the conserved, essential core of the microtubule nucleating machinery, and it is found in nearly all eukaryotes. γTuSC has two copies of γ-tubulin and one each of γ-tubulin complex protein 2 (GCP2) and GCP3 (see the figure, part a). In many eukaryotes, multiple γTuSCs assemble with GCP4, GCP5 and GCP6 into the γ-tubulin ring complex (γTuRC) (see the figure, part b). Previous models of γTuRC assembly suggested that GCP4, GCP5 and GCP6 together function as a cap-like scaffold for arranging multiple γTuSCs into a distinctive ring shape. This view depicts a model with six γTuSCs (12 γ-tubulins), which would leave a gap in the template, owing to the fact that microtubules are made up of 13 profilaments. The most widely accepted model for the mechanism of γTuRC-based nucleation, the template model, suggests that γTuRC acts as a template, presenting a ring of γ-tubulins that make longitudinal contacts with α-tubulin–β-tubulin (see the figure, part c). The protofilament model, on the other hand, suggests that the γTuRC unfurls to present a γ-tubulin protofilament, which would nucleate through lateral contacts with αβ-tubulin (see the figure, part d). A complete list of proteins that are thought to be part of γTuSC and γTuRC, including the more recently identified MOZART1 and MOZART2 proteins, are listed in part e of the figure, along with alternative names for each protein. The five GCPs share regions of homology, although with very low levels of sequence identity (as low as 15% identity between different GCP families). Two homologous regions, grip1 and grip2, initially defined the homology82 (see the figure, part f). Regions of more distant homology were later shown to be more widely dispersed in the GCP sequences32, 50 (green shading in part f of the figure).
