Figure 2. Bacterial community composition of the colonic mucosa throughout the antibiotic treatments.
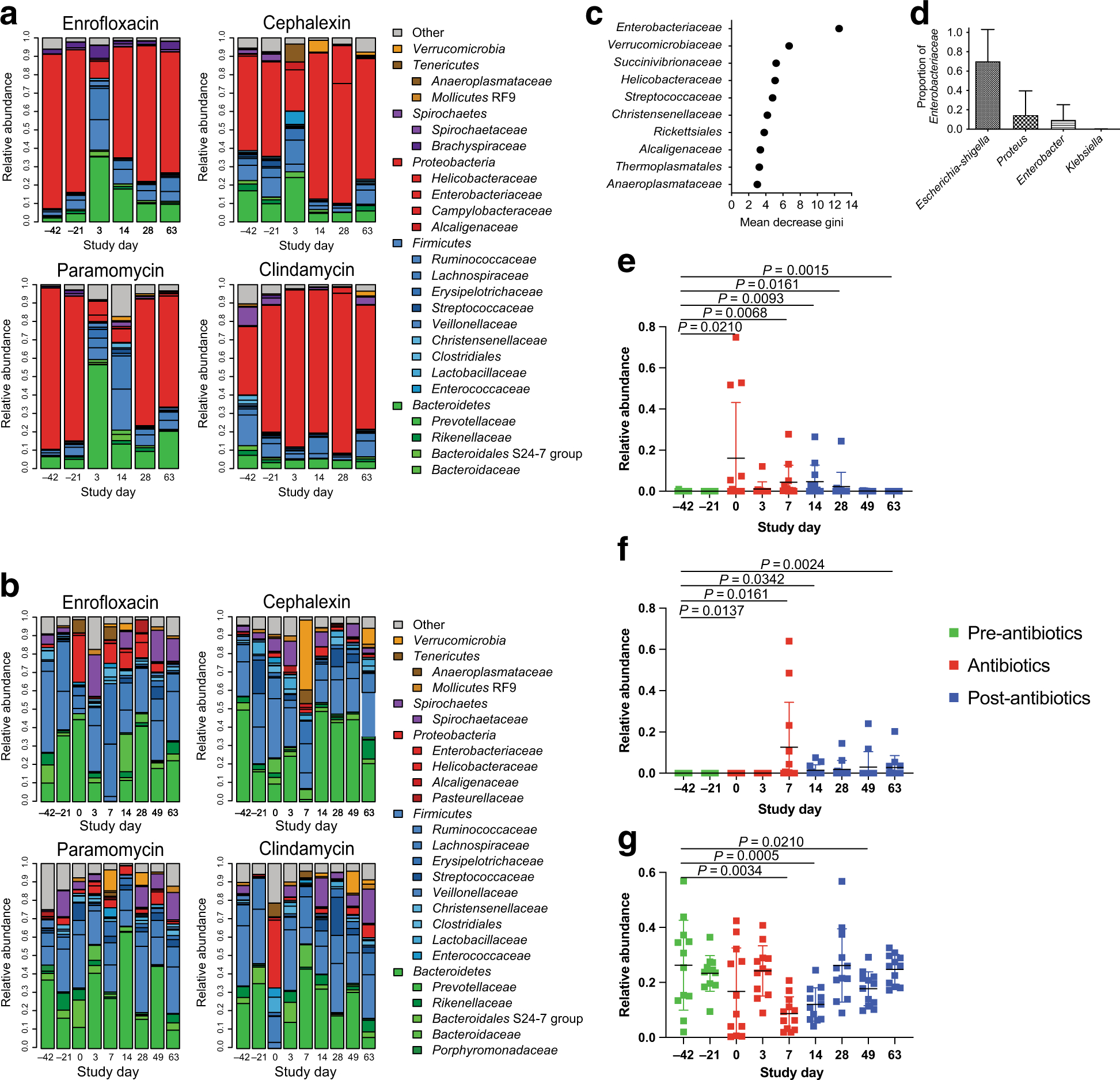
(A) Mucosal bacterial communities of all animals showed a high abundance of Helicobacteraceae prior to the antibiotic treatment. The antibiotics disrupted the mucosal bacterial communities, but clindamycin showed no impact on the predominant Helicobacteraceae. (B) Stool-associated bacterial communities of all of the animals were highly diverse, predominated by bacteria from the phyla Firmicutes and Bacteroides. Each antibiotic disrupted these communities, but the effects on community composition varied between the different antibiotics. (C) Random Forest analysis demonstrated that Enterobacteriaceae were the most strongly discriminating bacterial group in the stool bacterial communities at time points when antibiotics were being administered. (D) Other discriminating groups included Verrucomicrobiaceae, Helicobacteraceae, and Streptococcaeae, among others. The most abundant genera of Enterobacteriaceae included Escherichia, Proteus, and Enterobacter with a small proportion of Klebsiella. (E) The relative abundance of Enterobacteriaceae in the stool bacterial communities increased rapidly upon initiation of the antibiotic treatments (Day 0–7), persisted in some animals until Day 14, and then returned to pre-antibiotic levels by Day 49. (F) Akkermansia, detected in the stool bacterial communities, increased in relative abundance near the end of the antibiotic treatment and then persisted through the end of the study. (G) Ruminococcaceae displayed decreased relative abundance on Days 7 and 14, and increased back to pre-antibiotic levels on Day 28. (E-G) Green points indicate samples taken prior to the antibiotic treatment, red points indicate samples during the antibiotic treatment, and blue points indicate samples taken after cessation of the antibiotic treatment. Statistics were performed across all antibiotic treatment groups using a Wilcoxon signed-rank test and corrected for multiple comparisons using a post-hoc Bonferroni correction.
