Fig. 1. Structures of TRPC channels.
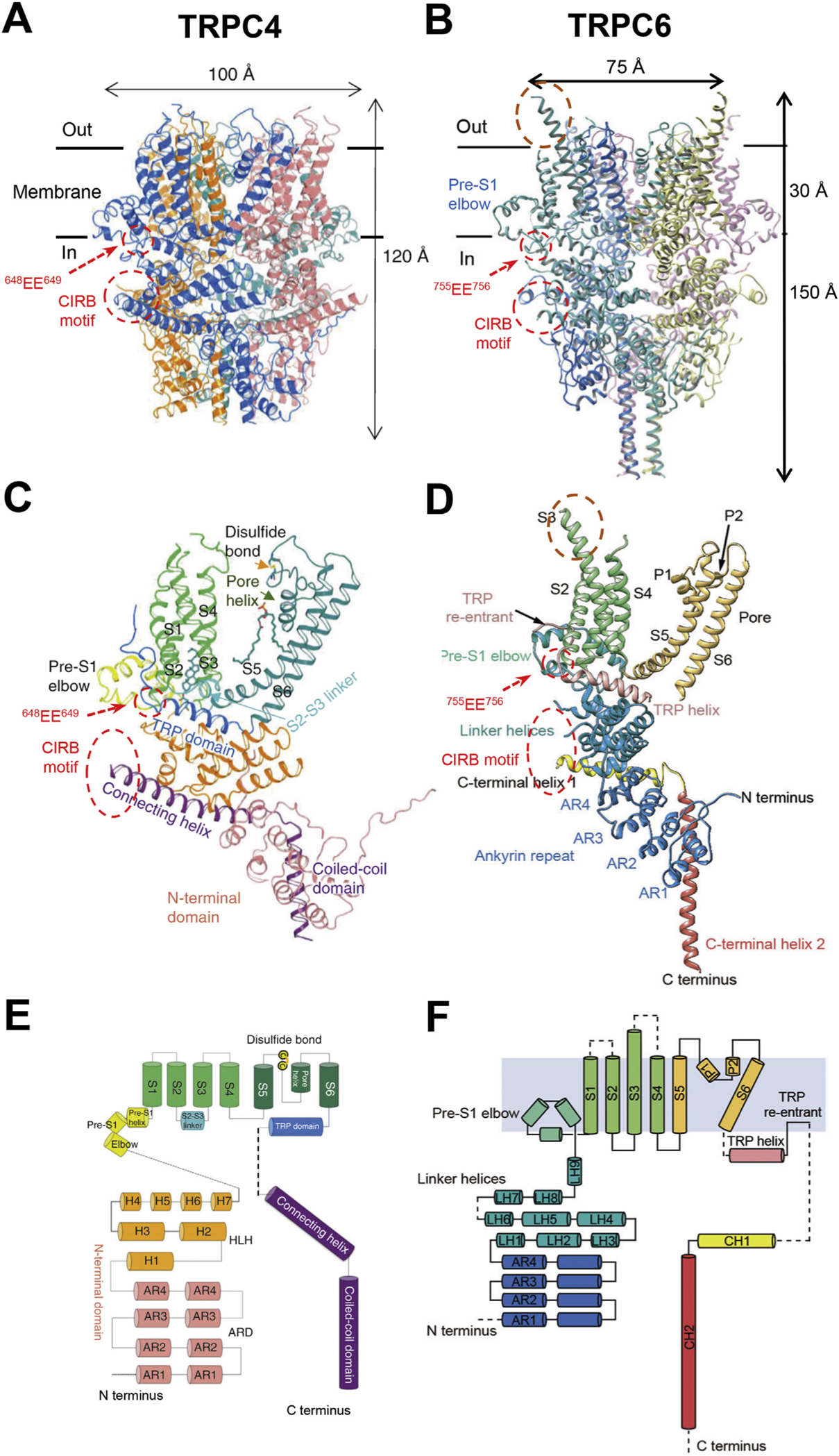
A & B, cryo-EM structures of C terminus truncated mouse TRPC4 (aa 1–758) (A) and full-length human TRPC6 (aa 1–931) (B), as reported by Duan et al. (2018) and Tang et al. (2018), respectively. The transmembrane regions are defined by the horizontal black lines with the thickness of about 30 Å. Areas of the Calmodulin- and IP3 receptor-binding (CIRB) motifs and the two conserved acidic residues (EE) critical for regulation by STIM1 are indicated by the dashed red circles. The extracellular protrusion of S3 transmembrane helix in TRPC6 is encircled by brown dashed line. Note the missing structures between the TRP re-entrant loop and CIRB motif in both examples. C & D, ribbon diagrams of single subunits of TRPC4 (C) and TRPC6 (D). TRP domain (in C) is equivalent to TRP helix (in D); Connecting helix and coiled-coil domain (in C) are equivalent to C terminal helices 1 and 2 (in D), respectively. E & F, topology and domain organization of TRPC4 (E) and TRPC6 (F) single subunits. Cylinders indicate α helices; dashed lines highlight unresolved structures.
