Fig. 3. Mechanism of activation of TRPC channels.
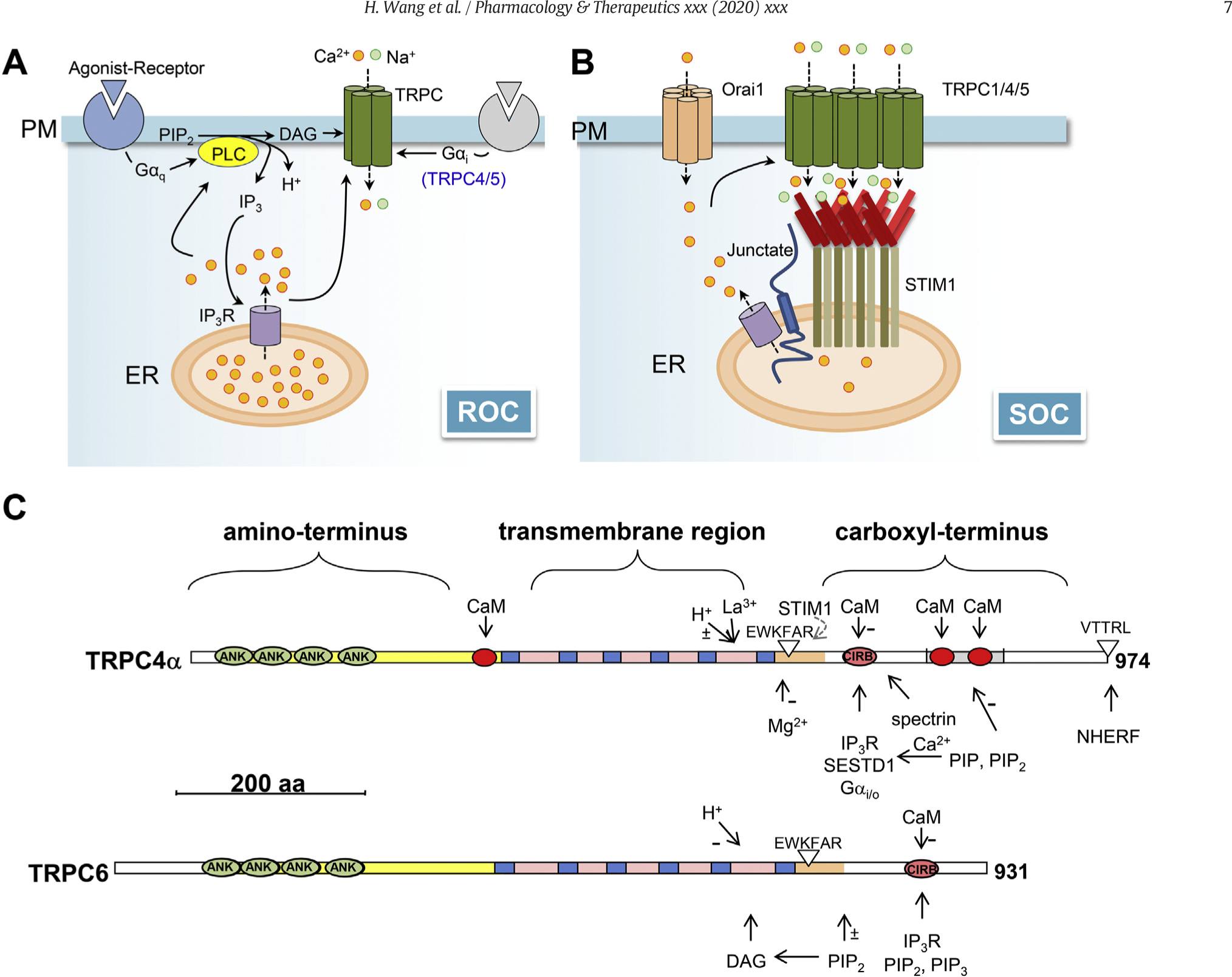
A, receptor-operated channel (ROC) activation of TRPCs. Independently of STIM1, receptor activation leads to PLC hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and production of inositol 1,4,5-trisphosphate (IP3), diacylglycerols (DAG), and protons (H+). All of them have been implicated in the regulation of TRPC channel function. In addition, IP3 activates IP3 receptors (IP3R) on the endoplasmic reticulum (ER) membrane to release stored Ca2+, causing [Ca2+]c to increase. Ca2+ regulates TRPC channels in both calmodulin (CaM)-dependent and -independent manners. Specifically for TRPC4 and C5, the activation of Gi/o proteins also triggers channel activation in conjunction with PLC stimulation. B, store (or STIM)-operated channel (SOC) activation of TRPCs. TRPC1/4/5 have been shown to be directly activated by STIM1 (see text for details), which senses ER Ca2+ store depletion to oligomerize and move towards the plasma membrane (PM). This may be facilitated by junctate, which interacts with IP3Rs, TRPCs, and STIM1, as well as by Ca2+ influx mediated by Orai1. C, linear representation of selected key structure features of TRPC4α (upper) and TRPC6 (lower). Ankyrin-like (ANK) repeats, transmembrane segments (blue boxes), and CaM-binding sites (red circles) are labeled. EWKFAR indicates the TRP motif. VTTRL indicates the PDZ-binding domain. Experimental evidence for some of the features indicated in TRPC4α has only been reported for TRPC5. −, experimental evidence exists for negative regulation; ±, experimental evidence exists for both positive and negative regulation. Binding motifs for NHERF (Tang et al., 2000), PIP2 and PIP3 (Kwon et al., 2007), SESTD1 (Miehe et al., 2010), Gi/oα-GTP (Jeon et al., 2012), STIM1 (Zeng et al., 2008), spectrin (Odell, Van Helden, & Scott, 2008), and Calmodulin- and IP3 receptor-binding (CIRB) site (Zhang et al., 2001; Tang et al., 2001) are indicated.
