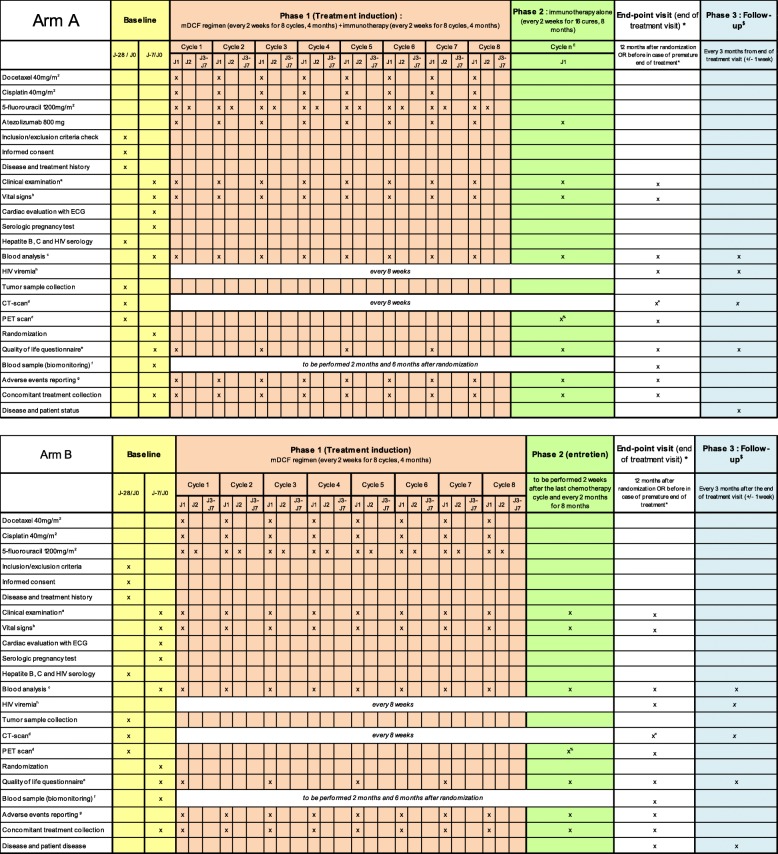
Fig. 1.
Schedule of enrolment, interventions, and assessments. a Clinical examination: height (at baseline only), weight, and ECOG-PS. b Vital signs: pulse, blood pressure and body temperature. c Blood analysis: complete blood count including red blood cells, haemoglobin, haematocrit, lymphocytes, white blood cells and differentials and platelets, blood electrolytes, bicarbonates, glycemia, proteinemia, albumin, blood urea nitrogen, creatinine, creatinine clearance (MDRD), calcium, magnesium, AST, ALT, gamma-GT, conjugated and total bilirubin, ALP, TSH, LDH, and C-reactive protein. d PET-scan at baseline, at first visit of phase 2 and at the end-point visit (except in case of early progression). CT-scans and PET-scans will be collected for a central review. e The EORTC-QLQC30 questionnaire: every second chemotherapy cycles in phase 1, and every 2 months during phase 2 until the end-point visit. f Blood samples for biomonitoring: 1 EDTA tube of 6 ml (plasma) and 8 EDTA tubes of 6 ml (PBMC). g According to the NCI-CTCAE guidelines version 4.03. h Only in HIV-positive patients. £ Immunotherapy alone: to be continued 1 cycle every to weeks up to a maximum of 12 months from the randomization date. * The end-point visit: to be performed 12 months after randomization or 4 weeks after the last chemotherapy cycle in case of premature end of treatment (for toxicity, progression, or patient or physician decision). $ The follow-up visit: to be performed every 3 months from the end-point visit to a patient death or at least 3 years after randomization