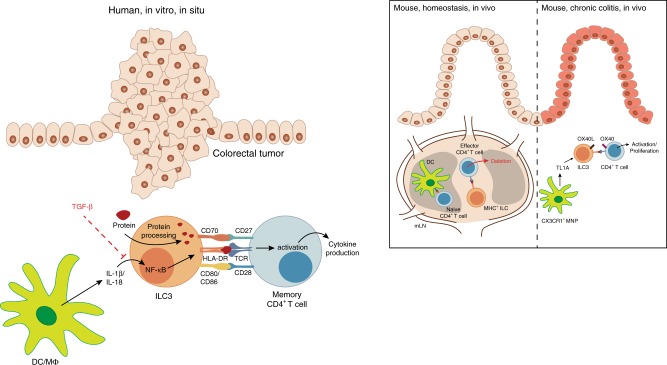
Fig. 8. Proposed model: the role of ILC3 in regulation of intestinal CD4+ T-cell responses.
While it is known that naive CD4+ T cells are primed in mesenteric lymph nodes (mLN), several studies demonstrated ILC3-based regulation of effector CD4+ T-cell responses in mice. MHCII+ ILC3 were shown to accumulate between T- and B-cell zones in mouse mLNs, co-localizing with emigrating commensal bacteria-specific effector CD4+ T cells and perpetrating the depletion of the latter20, 21. In the setting of experimental chronic colitis, TNF-like ligand 1A (TL1A) produced by mononuclear phagocytes (MNPs) was observed to induce OX40L expression on ILC3, which in turn promoted T-cell activation and immunopathology23. In the present study we show that ILCs in human colorectal tumors display increased HLA-DR expression and co-localize with T cells in situ. Further in vitro analysis revealed that the inflammasome-associated cytokines IL-1β and IL-18 could drive the expression of HLA-DR and co-stimulatory molecules on PB ILCs in an NF-κB-dependent manner, while TGF-β potently inhibited these antigen-presenting properties. IL-1β- and IL-18-activated ILCs were able to induce memory CD4+ T-cell responses in an autologous co-culture system following the uptake of full protein antigen. Hence, we would like to propose that cytokines present in the human colorectal tumor microenvironment might be able to modulate the antigen-presenting properties of intestinal ILCs with potential consequences for anti-tumor immune responses.