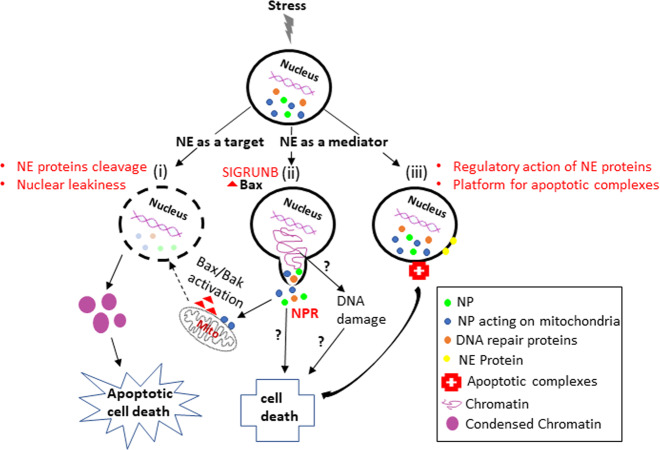
Fig. 4. The NE is both a target and a mediator of the apoptotic process.
Apoptotic stress converges on the NE where it promotes two major effects. (i) Caspase-dependent cleavage of NPC proteins and lamins. This in turn causes NE leakiness and entrance of caspases and nucleases from the cytosol to the nucleus, leading to NE breakdown and chromatin condensation and fragmentation. This nuclear destruction, along with additional non-nuclear events, culminate in apoptotic cell death. (ii–iii) Mediation of the death process. This effect includes (ii) Bax (red triangle)-dependent and caspase-independent transient SIGRUNB, which leads to NPR from the nucleus to the cytosol and perhaps also nuclear DNA damage. Some nuclear proteins such as nucleophosmin and histone 1.2 can translocate to the mitochondria and activate Bax/Bak, leading to MOMP. DNA repair factors are also released during SIGRUBN, depriving the nucleus of the ability to repair damage, further contributing to cell death. Other nuclear proteins released to the cytosol may also contribute to cell death by yet unknown mechanisms. (iii) Regulatory role of the NE on the apoptotic machinery. NE proteins can have pro- and anti-apoptotic effects and thus may regulate the apoptotic process. Furthermore, the NE can serve as a platform for the recruitment and assembly of apoptosis-promoting complexes. Uncertainties are indicated by a “?”.