Figure 1: MDSC inhibit FcR mediated NK cell effector functions and signal transduction.
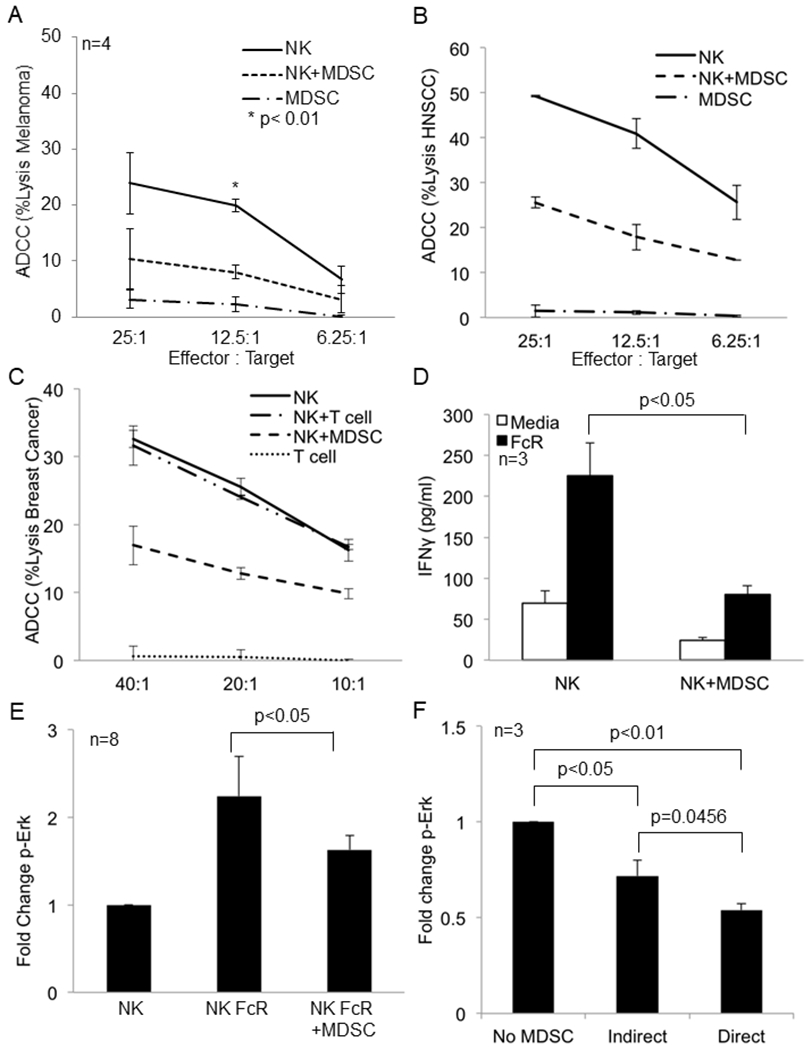
(a) NK cells from the peripheral blood of a melanoma patient were cultured alone or with autologous MDSC overnight and then used in a 51Cr release ADCC assay against cetuximab-coated HT-29 cells. Values represent the mean ± SD from four independent experiments, p<0.01. Significance was determined using the paired t-test. (b) Results from one ADCC assay as conducted in (a) using NK cells and autologous MDSC from a HNSCC patient. (c) Results from one ADCC assay as conducted in (a) using NK cells and autologous MDSC from a breast cancer patient. (d) Autologous NK cells and MDSC from the peripheral blood of melanoma patients were co-cultured at a 1:1 ratio in 96 well plates coated with human IgG (100 μg/ml) or media (control). Supernatants were collected after 48 hrs and cytokine levels measured by ELISA. Quantification of data from three independent experiments, values shown are the mean ± SE, p<0.05. Significance was determined using a paired t-test. (e) Quantification of changes in p-Erk levels in FcR activated CD56+ NK cells measured by flow cytometry in the presence or absence of MDSC. Values are mean ± SE from eight independent experiments, p<0.05. Significance was determined using a paired t-test. (f) NK cells were cultured alone, in direct contact with MDSC (Direct), or physically separated from MDSC by a permeable 0.4 μm Corning Transwell® membrane (Indirect) at a 1:1 ratio overnight. NK cells were then stimulated through the FcR using the anti-CD16 3G8 antibody and F(ab’)2 and levels of p-Erk determined as described above. Values are the mean ± SE from 3 independent experiments. Significance was determined using a paired t-test and Holms method. Representative flow cytometry dot plot for p-Erk is provided in Supplementary Figure 4.
