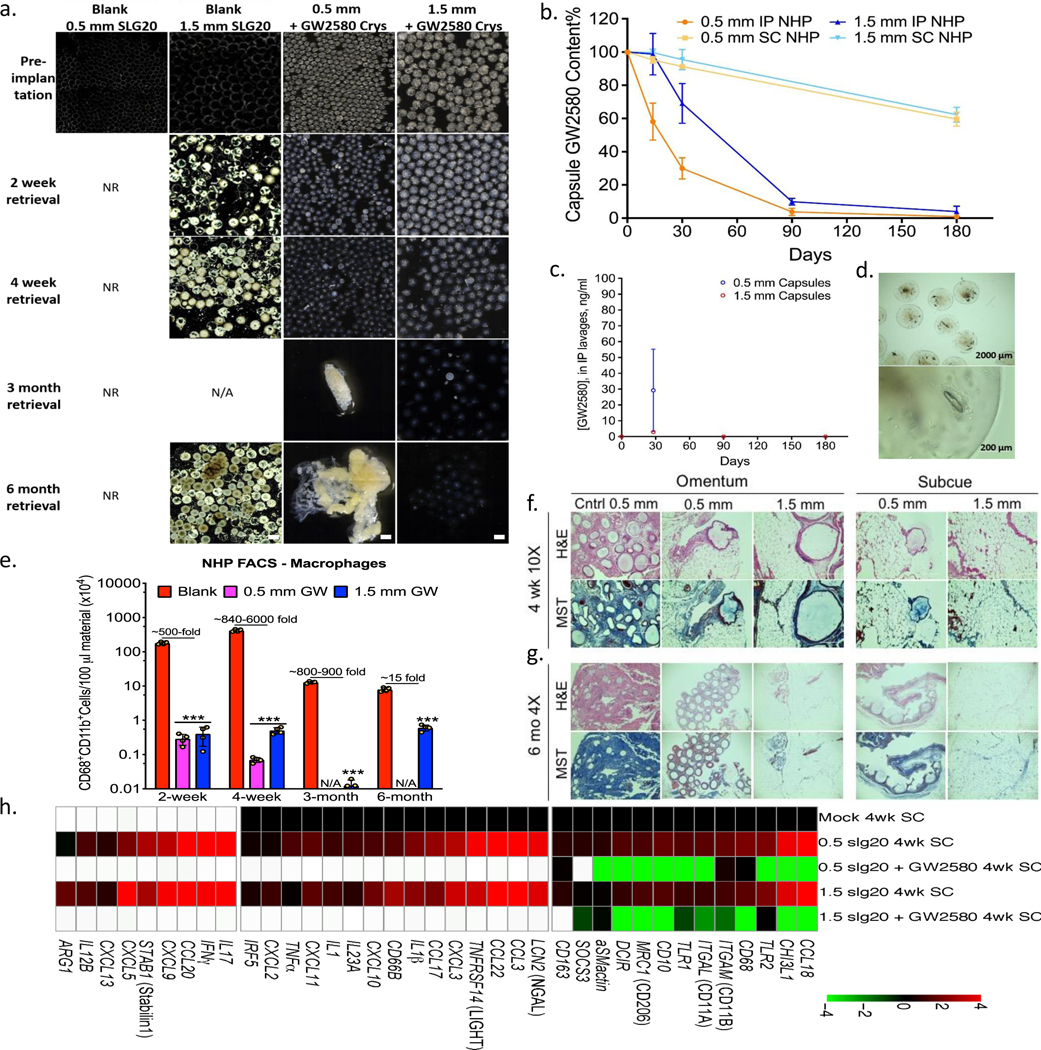
Figure 4. Long-term anti-fibrotic efficacy in non-human primates.
Crystalline GW2580 was tested in alginate spheres across different implant sites. a) Phase contrast images showing host FBR against 0.5 and 1.5 mm diameter alginate spheres encapsulating crystalline CSF1R inhibitor GW2580, after 2 and 4-week as well as 3 and 6-month intraperitoneal (IP) implantations in non-human primates (NHP); N=2/group. See Supplementary Fig. 12 for a full IP and SC implant panel over time. Note: drug crystals have colored appearance, making drug loaded-alginate capsules more opaque/white. NR=not-retrievable, N/A=not-available. Scale bars=1500μm. b) Drug extraction analysis (HPLC) revealed significant crystals quantities left inside retrieved capsules (up to 8% or 68% of initial loading for IP or SC capsules, respectively). c) Lavage GW2580 concentrations from the IP space surrounding 0.5 mm and 1.5 mm implanted capsules. d) Microscopy images showing retrieved 1.5 mm capsules after 6 months from IP space. Crystals were found to release in a gradual manner leaving hollow spaces. e) Quantitative FACS analysis of cells dissociated from alginate spheres, retrieved after various post-IP implantation times, as specified, vs. 1.5 mm blank SLG20 capsules. Macrophage presence is reduced with drug as compared to empty (control) spheres (log base 10 scale). H&E and Masson’s Trichrome stained histological sections of excised IP omentum or SC tissue 4 weeks (f) and 6 months (g) post-implant showing reduced fibrosis in various crystalline drug (GW2580) groups, vs. blank 0.5 mm control spheres (Scale bars=400μm (10X) or 1000 μm (4X), respectively). h) NanoString analysis of 4-week implanted SC tissues for immune markers and cytokines, as compared to mock (saline-injected) controls. White-within two standard deviations of assay’s mean background. All bar or line graph data:mean±SEM. Statistical analysis:one-way ANOVA plus Bonferroni multiple comparison correction; ***:p<0.0001. N=4 samples/group. All subpanels reflect representative data from in vivo experimental analyses repeated twice.