Extended Data Figure 3: The development of sugar preference.
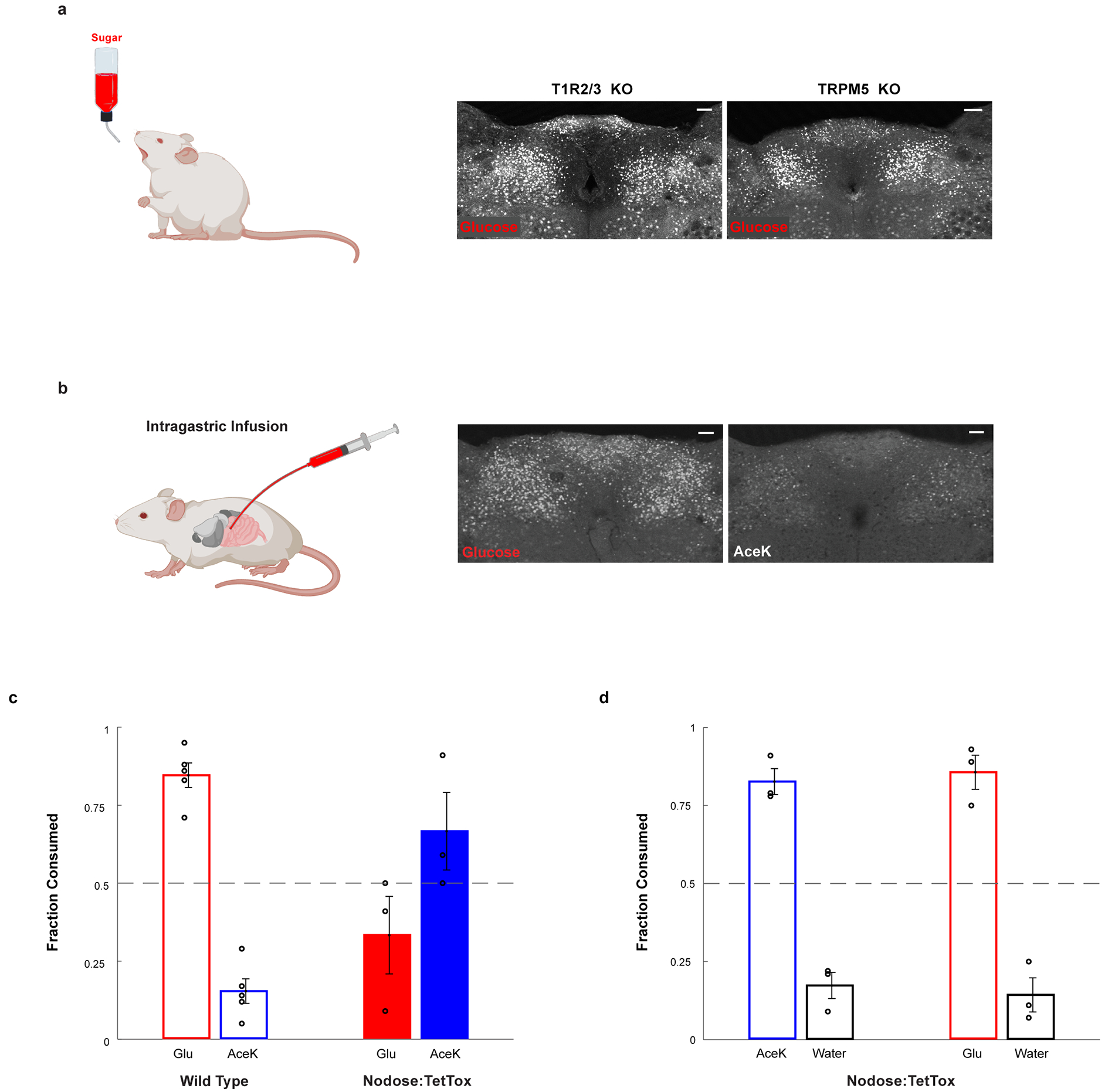
a, Glucose stimulates cNST neurons in animals lacking the sweet taste receptor (T1R2/T1R3−/−), or in animals lacking the TRPM5 ion channel (TRPM5−/−). See Fig. 1e for quantitation: T1R2/T1R3−/−, n = 5 mice, ANOVA followed by Tukey’s HSD post hoc test, p < 0.0001; TRPM5−/−, n = 7 mice, ANOVA followed by Tukey’s HSD post hoc test, p < 0.0001. Values are mean ± s.e.m. Scale bar, 100 μm. b, Direct intragastric infusion of glucose, but not AceK, robustly activates the cNST. n = 2 independent experiments. Scale bar, 100 μm. c-d, Genetic silencing of vagal sensory neurons. c, Sugar-preference graphs for Wild Type mice (n = 5 mice), demonstrating the robust development of preference for sugar versus artificial sweetener (see also Fig. 1). In contrast, silencing of the sensory neurons in the nodose ganglia, by bilateral injection of AAV DIO-TetTox injections into the nodose ganglia of VGlut2-Cre animals (see Methods), abolishes the development of sugar preference; n = 3 animals, two-sided Mann-Whitney U-Test, p = 0.035. Values are mean ± s.e.m. d, However, silencing vagal sensory neurons does not impair the innate attraction to “sweet” solutions; shown are behavioural responses to AceK versus water, and glucose versus water (n = 3 animals, preference index for AceK = 0.82, preference index for glucose = 0.85). Values are mean ± s.e.m.
