Figure 1. SIRT3 is important for normal wound healing.
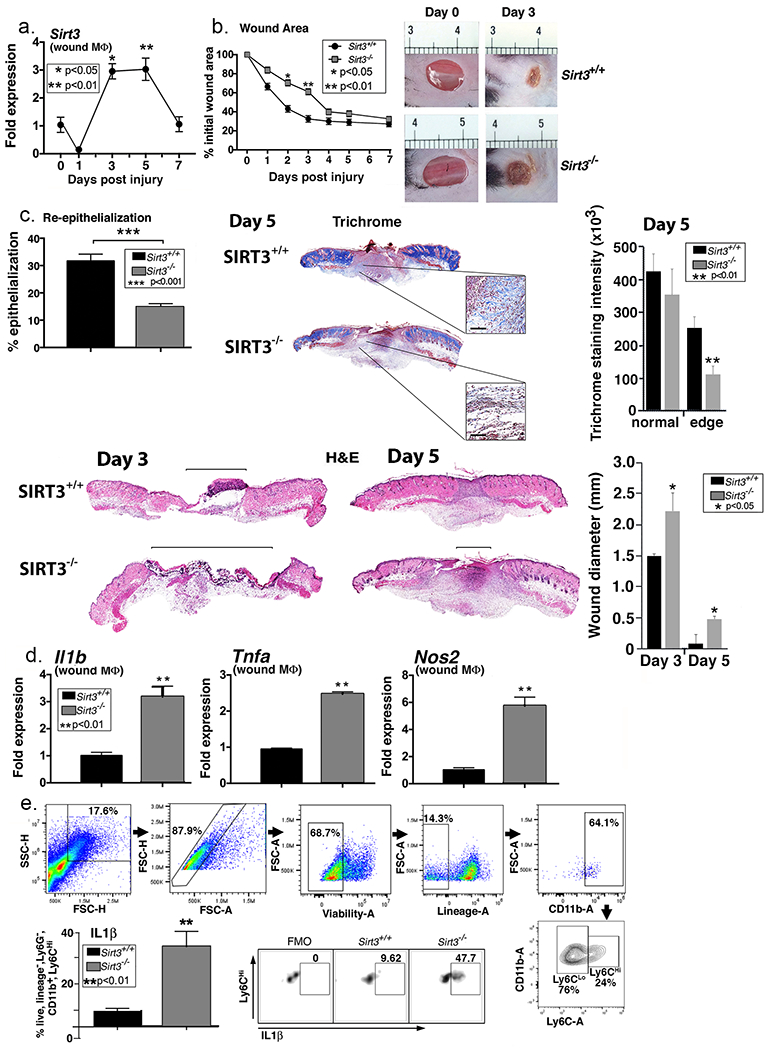
A. Two wounds were created using a 4mm punch on the backs of wild type C57BL/6 mice. Wound macrophages (CD11b+CD3− CD19−Ly6G−) were isolated at baseline (day 0) and various time points post-injury and Sirt3 gene expression was assessed (n=20 mice; repeated 2x). B: 4mm punch biopsies were created in Sirt3−/− mice and littermate controls (Sirt3+/+) and analyzed daily for changes in wound area using ImageJ software (n=10 mice; repeated 1x). Representative photographs of the wounds of on days 0 and 3 post-injury are shown. C: Wounds from Sirt3−/− and Sirt3+/+ controls were harvested on day 5, paraffin embedded and sectioned. Sections were selected at the wound center at the maximum wound diameter. 5 μm sections were stained with hematoxylin-eosin (H&E) and Masson’s Trichrome. Representative sections are shown. Percent re-epithelialization was calculated by measuring distance traveled by epithelial tongues on both sides of wound divided by total distance for full re-epithelialization. Wound diameter was calculated from and to the next leading epithelial wound edge across the maximum diameter of the wound. Black bar over H&E sections denotes wound diameter. Trichrome staining was calculated using ImageJ software (n=3 mice). Black scale bar in magnified blowout denotes 100 µm scale. D: Wound macrophages (CD11b+CD3− CD19−Ly6G−) were isolated from Sirt3−/− mice and Sirt3+/+ controls on day 3 post-injury. Relative gene expression of Il1b, Tnfa, and Nos2 was measured (n=8 mice; repeated 2x). E: Wounds were harvested from Sirt3−/− and Sirt3+/+ controls on day 3 and processed for ex vivo intracellular flow cytometry. The gating strategy used for intracellular flow cytometry selecting live, lineage− (CD3−CD19−Ter119−NK1.1−), non-neutrophil (Ly6G−), CD11b+ cells is shown. Conservative gates were chosen to limit including cells in early apoptosis. Flow cytometry quantification of Ly6C revealed two populations of cells in both Sirt3−/− and Sirt3+/+ wounds, translating to Ly6Chi and Ly6Clo monocyte/macrophages. Intracellular cytokine quantification by flow cytometry of IL1β in Ly6Chi wound monocyte macrophages is shown (n=10 mice, repeated 1x). Data are presented as mean ± SEM. Data were analyzed for normality and 2-test Student t-test was performed. For data with multiple comparisons, ANOVA followed by Newman-Keuls multiple comparisons test was performed.
