Figure 3. Sirt3 expression is significantly reduced in diabetic mice compared with controls.
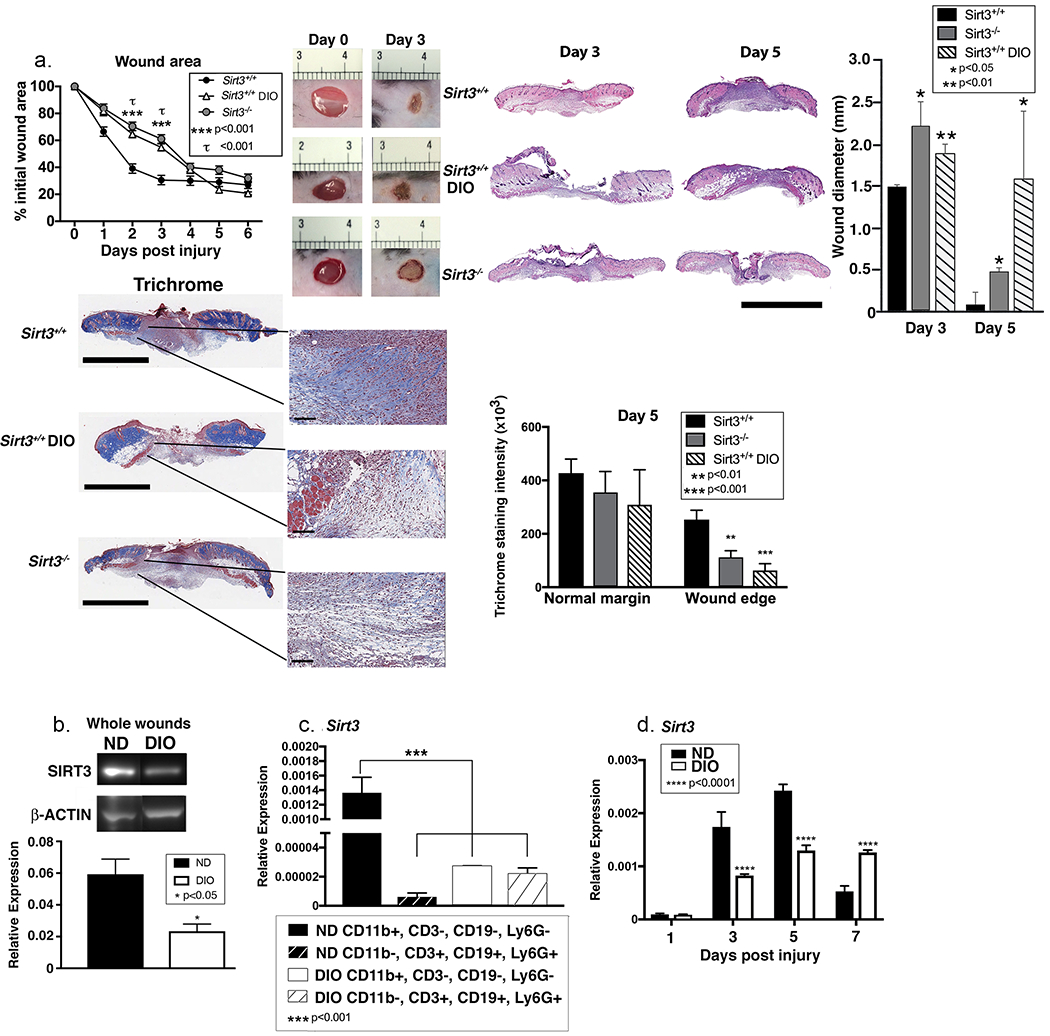
A: Wounds were created using 4 mm punch biopsies on the backs of DIO, Sirt3−/− mice and littermate controls (Sirt3+/+). Change in wound area was analyzed daily using ImageJ software (n=10 mice; repeated 2x). Wounds from DIO, Sirt3−/− mice and wild-type littermate controls (Sirt3+/+) were harvested on days 3 and 5, paraffin embedded and sectioned. Sections were selected at the wound center at the maximum wound diameter. 5 μm sections were stained with hematoxylin-eosin (H&E) and Masson’s Trichrome. Representative sections are shown. Scale bars represent 2mm (trichrome) or 3mm (H&E) for low magnification images and 100µm for blowouts (trichrome). Wound diameter was calculated from and to the next leading epithelial wound edge across the maximum diameter of the wound. Trichrome staining was calculated using ImageJ software (n=3 mice). B: Whole wounds were harvested from DIO mice and normal diet controls on day 3 post-injury. SIRT3 protein expression was assessed using Western Blot (n=8 mice; repeated 4x). C: Wound macrophages (CD11b+CD3−CD19−Ly6G−) or CD11b−CD3+CD19+Ly6G+ cells were isolated from DIO mice and controls on day 3 post-injury by cell sorting. Sirt3 gene expression was measured. (n=10 mice, repeated 2x). D: Wound macrophages (CD11b+CD3−CD19−Ly6G−) were isolated from DIO mice and controls on days 1, 3, 5, and 7 post-injury by cell sorting. Sirt3 gene expression was measured. (n=10 wounds, repeated 2x). Data are presented as mean ± SEM. Data were analyzed for normality and 2-test Student t-test was performed. For data with multiple comparisons, ANOVA followed by Newman-Keuls multiple comparisons test was performed.
