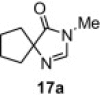
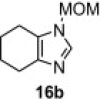
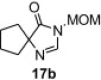
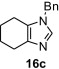
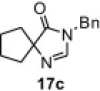
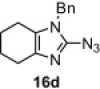
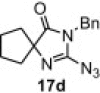
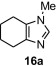Table 8.
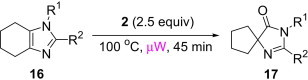
Isolated yield.
Solution-phase oxidative rearrangement using 2-benzenesulfonyl-3-phenyloxaziridine (2 equiv), CHCl3 at room temperature for 4 h (Ref. 39).
Isolated yield when the oxidative rearrangement was carried out with polymer 1 (2 equiv) at room temperature for 24 h.
Solution-phase oxidative rearrangement using 2-benzenesulfonyl-3-phenyloxaziridine (2 equiv), CHCl3 at 35 °C for 24 h (Ref. 39).
Isolated yield when the oxidative rearrangement was carried out with polymer 1 (2.5 equiv), 100 °C, μW, 45 min.
Solution-phase oxidative rearrangement using 2-(phenylsulfonyl)-3-(p-nitrophenyl)oxaziridine (2 equiv), CHCl3 at 35 °C for 24 h (Ref. 39).