Fig. 2.
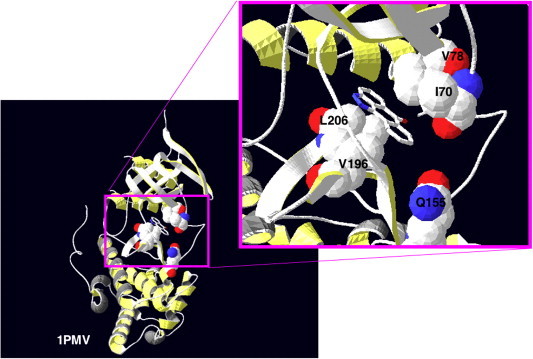
Structure of the JNK complex with the ATP-competitive inhibitor SP600125. SP600125 has been co-crystallised with JNK3, and the resulting structure has been recorded in the Protein DataBase (PDB) as 1PMV [5]. Here, the residues in JNK3 not conserved in p38-2, namely I70, V79, V196, L206 and Q155, are highlighted. Although these residues were predicted to most likely contribute to the specificity of SP600125 towards JNK1/2/3 over the p38 MAPKs [5], subsequent mutagenesis studies further work is required to evaluate which residues make major contributions to binding [6].
