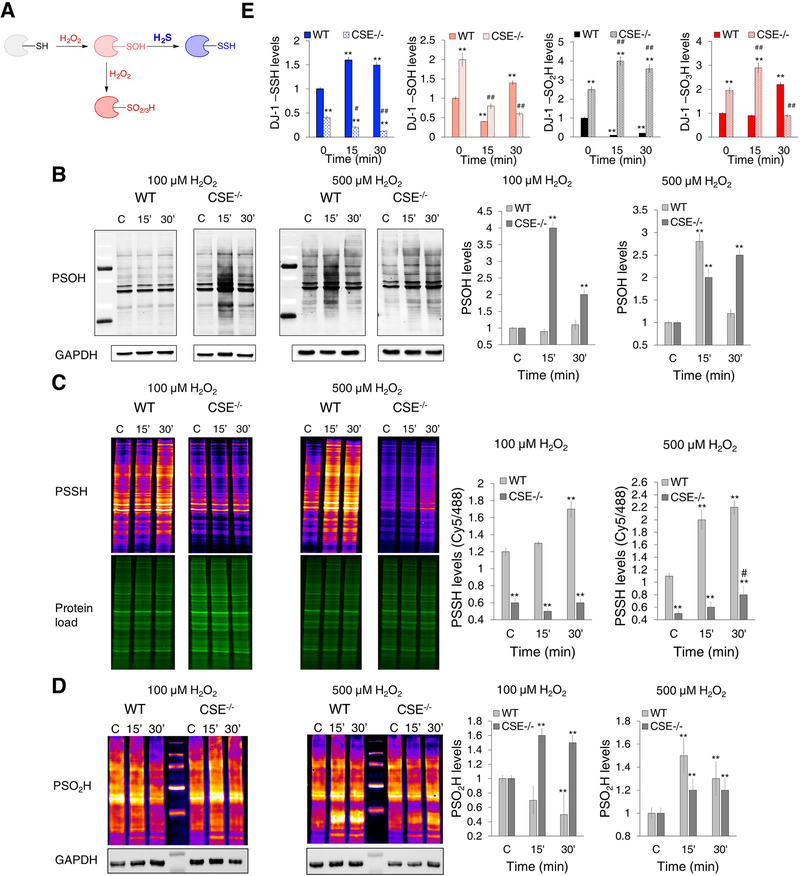
Figure 4. Endogenous H2S controls cysteine oxidation caused by H2O2.
(A) The proposed mechanism for the redox switching between H2O2-induced thiol oxidation and persulfidation.
(B-D) Cysteine oxPTM levels in WT and CSE−/− MEF cells treated with 100 or 500 μM H2O2 for 15 and 30 min. (B) Protein sulfenylation (PSOH) (labeled with DCP-Bio1 and visualized with streptavidin-488). GAPDH was used as a loading control. n = 4. (C) Protein persulfidation (PSSH) (labeled with DAz-2:Cy5 as a switching agent). Ratio of Cy5/488 signals is used for the quantification. n = 3. (D) Protein sulfinylation (PSO2H) (labeled with BioDiaAlk and visualized with streptavidin-Cy5). GAPDH was used as a loading control. n = 5. PSOH and PSSH values were normalized to the levels found in untreated cells. ** p < 0.01 compared to the untreated WT cells; # p < 0.05 compared to the untreated CSE−/− cells.
(E) Persulfidation, sulfenylation, sulfinylation and sulfonylation of DJ-1. WT and CSE−/− MEF cells were treated with 100 μM H2O2 for 15 or 30 min, labeled for PSSH, PSOH and PSO2H, immunoprecipitated with anti-DJ-1 antibody immobilized to agarose beads and immunoblotted with anti-biotin antibody. For sulfonylated DJ-1 (DJ-1-SO3H), antibody selective for C106 sulfonic acid of DJ-1 was used. n = 4. ** p < 0.01 vs. untreated WT. # p < 0.05, ## p < 0.01 vs. untreated CSE−/− cells.