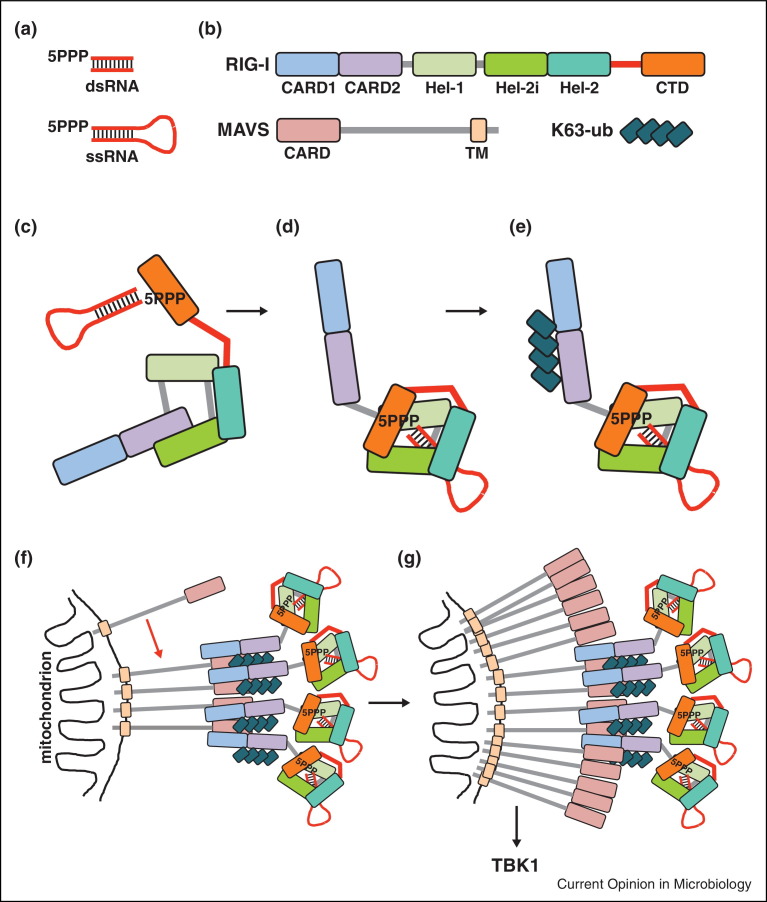
Figure 2.
Model of RIG-I activation and signalling. (a) The most potent RIG-I agonists are characterized by a 5PPP moiety and by base-pairing to a complementary stretch of RNA. This base-pairing can either be provided by a second molecule of RNA or by complementarity of the 5′-end and 3′-end of the 5PPP bearing RNA. As such, RIG-I agonists can be double-stranded (ds) and single-stranded (ss) RNAs. (b) The domain architecture of RIG-I and MAVS is shown schematically. CARD, caspase recruitment domain; CTD, C-terminal domain; TM, transmembrane domain; Hel-1, Hel-2i and Hel-2, subdomains of the RIG-I helicase domain; K63-ub, K63-linked polyubiquitin. The RIG-I pincer domain (also called bridging helices) is shown as a red line connecting the helicase domain and CTD. (c) In the autorepressed conformation, the CTD is flexibly connected to the helicase domain and this allows for binding of 5PPP groups of viral RNA genomes to the CTD. (d) Upon RNA binding to the CTD, the helicase domain makes contacts with the RNA and RIG-I undergoes a conformational change that exposes the CARDs. (e) RIG-I is then ubiquitylated or binds to free ubiquitin chains. (f and g) This facilitates RIG-I tetramerization and interaction with MAVS. Upon initial oligomerization of MAVS, a prion-like mechanism recruits additional MAVS molecules into the complex (red arrow) and signal transduction is initiated via TBK1 activation.