Highlights
-
•
Bronchiolitis by respiratory syncytial virus is more severe than by rhinovirus.
-
•
NA1 genotype had major hospitalization rate, severe disease and higher viral load.
-
•
Co-infection by RSV and HRV does not increase the severity of respiratory illness.
Abbreviations: RSV, respiratory syncytial virus; HRV, rhinovirus; ALTRI, acute low tract respiratory infection; RT-PCR, reverse transcription-polymerase chain reaction; hMPV, human metapneumovirus; NPA, nasopharyngeal aspirates; IFA, indirect immunofluorescence assay
Keywords: Respiratory syncytial virus, Rhinovirus, ALTRI, Severe infantile respiratory infection
Abstract
Background
Respiratory syncytial virus (RSV) and rhinovirus (HRV) are the main cause of acute lower respiratory tract infections (ALRTIs) in infants. Viral and host-related risk factors for severe disease have also not been clearly established.
Objective
To assess whether certain viral features of RSV and, or HRV are associated with severe ALRTI.
Study design
RSV and HRV were studied in nasopharyngeal samples of infants by immunofluorescence, Luminex® and/or real-time RT-PCR assays. Quantitation and genotyping of RSV and HRV by PCR were done.
Results
Of 124 virus positive specimens, 74 (59.7%) had RSV; 22 (17.7%) HRV and 28 (22.6%) RSV-HRV co-infection. Hospitalization was required in 57/74 RSV infants (77.0%); in 10/22 HRV cases (45.5%) (p = 0.006) and in 15/28 co-infected by both viruses (53.6%) (p = 0.003). Severe cases were 33/74 (44.6%) RSV infections, 2/22 HRV cases (9.1%), (p < 0.002) and 6/28 (21.4%) patients co-infected by RSV–HRV (p < 0.026). Three genotypes (NA1, B7, B9) of RSV circulated during the study. In 33 severe infants, NA1 was detected in 19 cases (57.6%); B7 in 13 (39.4%) and B9 in 1 (3.0%) (p < 0.01; OR = 10.0). RSV loads were similar between outpatients and hospitalized infants (p = 0.7) and among different severities (p = 0.7). NA1 loads were higher than other strains (p = 0.049). Three geno-groups of HRV circulated homogeneously.
Conclusion
In very young infants, RSV cause more severe disease than HRV. Co-infection does not increase the severity of illness. NA1 RSV genotype was associated with major frequency of hospitalization, severe respiratory disease and higher viral load.
1. Background
RSV is a leading cause of acute lower respiratory tract infections (ALRTI) in infants [1], [2], [3]. In Chile, RSV is the major cause of ALRTI causing yearly winter outbreaks with a fatality rate below 0.1% [4], [5]. Rhinovirus (HRV) is the main agent of common cold and his prevalence in ALRI has been underestimated for many years. Recent studies have demonstrated that HRV is a cause of bronchiolitis, as common as RSV, with frequencies of 16 and 18% [6], [7].
Clinical features for both viruses, RSV and HRV, may be indistinguishable. Some authors reported that children hospitalized with HRV were older than those infected with RSV and its association with atopy is higher than that recorded in RSV infections [6], [8].
Viral and host-related risk factors for severe disease have not been clearly established. Infants younger than 6 months of age are the major risk group for RSV severe disease and some authors have suggested that co-infections with HRV can lead to severe illness [6], [7], [8], [9], although anti-inflammatory response is different among HRV and RSV infection [10], [11], [12], [13].
2. Objective
The aim of this study was to assess whether certain viral features of RSV and, or HRV are associated with severe ALRTI in Chilean infants younger than 6 months of age, according to a clinical score.
3. Methods
3.1. Subjects
Previously healthy term infants, younger than 6 months of age, with a normal weight at birth, having their first acquired-community ALRTI, were consecutively enrolled into the study during the winter seasons of 2010 and 2011 from the Cruz-Melo outpatient clinic and the Roberto del Rio Hospital, in the northern area of Santiago de Chile. Infants were enrolled during the first three days of respiratory symptoms (nasal discharge, cough or respiratory distress). ALRTI was confirmed by clinical signs of respiratory distress with crackles or wheezing, or hyperinflation in a chest radiograph. Exclusion criteria for patients were: (i) previous hospitalization for any cause, (ii) primary or secondary immunodeficiency, (iii) prematurity, (iv) bronchopulmonary dysplasia, (v) previous mechanical ventilatory support, (vi) congenital heart disease, and (vii) any previous respiratory disease, including common cold and otitis media.
3.2. Severity of ALRTI
The severity of ALRTI was classified according to a previously published scoring system [14]. A score of ≥7 points identifies severe infants; 4–6 points indicate a moderate disease and 0–3 points, a mild illness.
3.3. Samples collection
A nasopharyngeal sample (NPS) at enrollment was collected by Copan® swab in 5 ml of Hank's solution during the first three days of respiratory symptoms.
3.4. Viral studies
Immunofluorescent assay (IFA) for RSV, influenza, parainfluenza, metapneumovirus and adenovirus were conducted immediately as described elsewhere.
Nasopharyngeal samples were tested using xTAG RVP FAST by Luminex® and Abbott Molecular®, according to the manufacturer's instructions for RSV, enterovirus/rhinovirus, influenza, parainfluenza, metapneumovirus, adenovirus, coronavirus and bocavirus.
For real time PCR, total RNA was extracted from NPA by the guanidinium thiocyanate–phenol–chloroform method [15]. First-strand cDNA was synthesized using 5 μl of viral nucleic acid, random hexamer primers and MMLV-RT (Promega®), in a Perkin Elmer Gene Amp® PCR System 2400 during 1 h to 37 °C. A fragment of the N gene of RSV was amplified with specific primers [16] by real time PCR in a Light Cycler 1.5 (Roche®). The 5′NCR of HRV was amplified using primers and conditions previously described, resulting in a product of approximately 400 bp [17].
RSV infection was defined by positive IFA and, or PCR and, or Luminex and HRV infection by positive PCR and, or Luminex.
For sequencing of PCR products and phylogenetic analyses for RSV and HRV, the most variable region of the G gene of RSV was amplified with 10 pmol of each primer FV and GAB [18], 2.5 U Taq DNA polymerase (Promega®) and 10 μl cDNA. The reaction was carried out for 5 min at 94 °C and for 35 cycles at 94 °C for 1 min, 55 °C for 1 min and 72 °C for 1 min and followed by 10 min of extension at 72 °C. Amplified products of 653 bp of RSV A and 656 bp of RSV B were visualized using ethidium bromide under UV light on a 1.5% agarose gel [16]. When required, semi nested PCR was performed with GAB and F1 primers [18] resulting in 489 bp and 492 bp products, depending on genotype.
PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN®) and FavorPrep™ Gel/PCR Purification Kit (Favorgen-Biotech Corp.). RSV and HRV amplicons were sequenced in both directions by Macrogen, Inc. Multiple sequence alignments were performed using the Clustal W program. Sequence alignment and phylogenetic analyses were performed with MEGA5 software [19], including reference sequences obtained from GenBank database (NCBI). The distance matrix analysis was conducted using the Kimura 2-parameter model and phylogenetic tree was constructed using neighbor joining method. Confidence of clustering of sequences was evaluated by bootstrapping (1000 replicates). Sequences were assigned to RSV genotype if they clustered with a significant bootstrap value >70% [20] and HRV with a value >50% [21]. RSV phylogenetic analyses were performed using 270 nucleotides sequences, including reference strains from all published RSV A and RSV B genotypes. HRV sequences comprised a 320 nucleotides region spanning the 5′ NCR, including 117 previously published sequences.
For quantitation of RSV genomes a quantitative real time RT-PCR was designed. Synthetic RNA was obtained by in vitro transcription after cloning of the N gene in pGEM-T-Easy vector (PROMEGA®). After purification, dilutions of RNA were used as quantitative standards during real time RT-PCR. The RT reaction was performed with specific oligonucleotide N1 (Table 1 ) and MMLV-RT during 1 h at 42 °C. Amplification was made with primers N1b and N2 and the N-Probe was included. An initial denaturation at 95 °C for 10 min was followed by 45 cycles of amplification (95 °C, 10 s; 58 °C, 10 s; 72 °C, 5 s) collecting fluorescence data on each cycle at 72 °C.
Table 1.
Oligonucleotides used for Respiratory syncytial virus genomes quantitation.
| Oligo | Sequence | Purpose |
|---|---|---|
| Primer N1 | 5′-GGAACAAGTTGTTGAGGTTTATGAATATGC-3′ | Reverse transcription |
| Primer N1b | 5′-CTACCATATATTGAAYAAYCCAAARGCATC-3′ | Amplification |
| Primer N2 | 5′-CTTCTGCTGTCAAGTCTAGTACACTGTAGT-3′ | Amplification |
| N-Probe | 5′-6FAM-CT + AGGC + AT + A + ATGGG + AGAATA-BBQ-3′ | Fluorescent detection |
3.5. Statistical analysis
Qualitative and quantitative data were compared between infants groups according to viral results by chi-square and Mann–Whitney Rank Sum test, respectively. Chi-square was also used for comparison of patients according to viral results, genotypes and severity of illness. Statistical analyses were performed using the SigmaStat (3.5) and EPI-Info-7 programs; p < 0.05 was considered significant.
4. Results
4.1. Subjects, severity and viral detection
A total of 139 infants were enrolled in the study and none of them died. Fifteen patients were excluded because they were negative for viruses or infected by other viruses. Of 124 included patients, 74 (59.7%) were infected by RSV as a single infection; 22 (17.7%) had HRV and 28 (22.6%) both viruses (Table 2 ); 80 (64.5%) were hospitalized; 41 (33.1%) had severe, 33 (26.6%) moderate and 50 (40.3%) mild illness.
Table 2.
Demographic features of the study subjects.
| Subject group | Number of cases | Inpatients | Outpatients | Gender |
Age (month) | p-Valuea | |
|---|---|---|---|---|---|---|---|
| n | n | M | F | Mean ± SE | |||
| RSV group | 74 |
57 |
17 |
31 |
43 |
2.236 ± 0.186 |
|
| Severe RSV group | 33 | 33 | 0 | 16 | 17 | 2.379 ± 0.322 | 0.9 |
| Moderate RSV group | 19 | 17 | 2 | 7 | 12 | 2.184 ± 0.377 | |
| Mild RSV group |
22 |
7 |
15 |
8 |
14 |
2.068 ± 0.242 |
|
| HRV group | 22 |
10 |
12 |
15 |
7 |
2.750 ± 0.42 |
|
| Severe HRV group | 2 | 2 | 0 | 0 | 2 | 1.000 ± 0.00 | 0.2 |
| Moderate HRV group | 4 | 4 | 0 | 3 | 1 | 4.000 ± 0.577 | |
| Mild HRV group |
16 |
4 |
12 |
12 |
4 |
2.656 ± 0.53 |
|
| RSV–HRV group | 28 |
13 |
15 |
14 |
14 |
3.071 ± 0.381 |
|
| Severe RSV-HRV group | 6 | 6 | 0 | 4 | 2 | 3.167 ± 0.703 | 0.3 |
| Moderate RSV-HRV group | 10 | 6 | 4 | 5 | 5 | 2.200 ± 0.359 | |
| Mild RSV-HRV group |
12 |
1 |
11 |
5 |
7 |
3.750 ± 0.730 |
|
| Total | 124 | 80 | 44 | 60 | 64 | 2.609 ± 0.149 | |
p-Values were obtained comparing age among severe, moderate and mild subgroups for each viral group (RSV, HRV and RSV–HRV) by Mann–Whitney Rank Sum test. RSV: Respiratory syncytial virus; HRV: Rhinovirus.
Among 74 RSV cases, 17 (23.0%) were outpatients and 57 (77.0%) required hospitalization. Of 22 infants with HRV, 12 (54.5%) were outpatients and 10 (45.5%) hospitalized. Thirteen of 28 children (46.4%) co-infected by both RSV and HRV were outpatients and 15 (53.6%) were hospitalized. Significantly more children were hospitalized with RSV infection than those infants infected with HRV (p < 0.007) or co-infected with both viruses (p < 0.04). No differences were observed in gender (p > 0.09) nor age (p > 0.1) between RSV, HRV and co-infected infants.
Severe cases were significantly more frequent in RSV infected patients (33/74, 44.6%) than HRV infants (2/22 = 9.1%) (p = 0.002) and cases with RSV and HRV (6/28, 21.4%) (p = 0.04). By contrast, infants infected with HRV showed a milder disease (16/22, 72.7%) than those with RSV (22/74, 29.7%) (p < 0.001) and co-infected patients 12/28 (42.9%) (p = 0.04). Moderate disease cases were similar among groups: 19/74 (25.7%) infected by RSV; 4/22 (18.2%) with HRV and 10/28 (35.7%) cases with both viruses (p > 0.3) (Table 2, Table 3 ).
Table 3.
Clinical features and maximal clinical requirement of the study subjects.
| Clinical features | Number of cases | Clinical outcome at discharge |
p-Valuea | ||
|---|---|---|---|---|---|
| Groups | 124 | Severe | Moderate | Mild | |
| RSV group | 74 |
n = 33 |
n = 19 |
n = 22 |
|
| Supplemental oxygen requirement | 52 | 33 | 19 | 0 | <0.001. |
| Critical care unit | 18 | 18 | 0 | 0 | <0.001 |
| Mechanical ventilation |
16 |
16 |
0 |
0 |
<0.001 |
| HRV group | 22 |
n = 2 |
n = 4 |
n = 16 |
|
| Supplemental oxygen requirement | 2 | 2 | 0 | 0 | <0.001 |
| Critical care unit | 0 | 0 | 0 | 0 | – |
| Mechanical ventilation |
0 |
0 |
0 |
0 |
– |
| RSV–HRV group | 28 |
n = 6 |
n = 10 |
n = 12 |
|
| Supplemental oxygen requirement | 16 | 6 | 10 | 0 | <0.001. |
| Critical care unit | 4 | 4 | 0 | 0 | <0.001 |
| Mechanical ventilation |
4 |
4 |
0 |
0 |
<0.001 |
| Clinical requirementb | Number of cases | Severe | Moderate | Mild | p-Valuec |
|---|---|---|---|---|---|
| RSV group | 74 |
n = 33 |
n = 19 |
n = 22 |
|
| Length of hospital stay (days) | 11.09 ± 0.93 | 4.32 ± 0.34 | 2.35 ± 0.35 | <0.001 | |
| Length of supplemental oxygen (days) | 8.94 ± 0.86 | 2.47 ± 0.23 | 0.00 ± 0.00 | <0.001 | |
| Maximal FIO2 administrated (%) |
45.14 ± 3.76 |
27.47 ± 0.32 |
21.00 ± 0.00 |
<0.001 |
|
| HRV group | 22 |
n = 2 |
n = 4 |
n = 16 |
|
| Length of hospital stay (days) | 11.50 ± 4.50 | 3.50 ± 0.50 | 1.12 ± 0.47 | <0.006 | |
| Length of supplemental oxygen (days) | 8.60 ± 4.50 | 2.00 ± 0.41 | 0.00 ± 0.00 | <0.001 | |
| Maximal FIO2 administrated (%) |
28.00 ± 0.00 |
27.25 ± 0.75 |
21.00 ± 0.00 |
<0.001 |
|
| RSV–HRV group | 28 |
n = 6 |
n = 10 |
n = 12 |
|
| Length of hospital stay (days) | 17.17 ± 4.36 | 4.00 ± 0.42 | 1.83 ± 0.62 | <0.001 | |
| Length of supplemental oxygen (days) | 15.67 ± 4.41 | 2.30 ± 0.33 | 0.00 ± 0.00 | <0.001 | |
| Maximal FIO2 administrated (%) | 54.75 ± 16.93 | 28.30 ± 0.88 | 21.00 ± 0.00 | <0.001 |
p-Values were obtained comparing clinical features among severe, moderate and mild patients for each group by Chi-square.
Data are geometric mean ± SE value.
Comparison of clinical requirement among severe, moderate and mild patients for each group by Mann–Whitney Rank Sum test. RSV: Respiratory syncytial virus; HRV: Rhinovirus.
Clinical features and requirements for each viral group were distributed significantly according to clinical outcome (Table 3).
4.2. RSV genotypes
Typification was performed in 33 RSV cases (Table 4 ); 14 (42.4%) of them were from outpatients and 19 (57.6%) in-patients. Among 33 genotipified RSV, 19 were NA1 genotype (57.6%) belonging to the group A; 13 were B7 (39.4%) and 1 was B9 (3.0%). Both genotypes belong to the group B. The NA1 strains were significantly more frequent in hospitalized infants (p < 0.001) and in severe disease (p = 0.01; OR = 10.0). This difference is mainly supported among infants with severe and mild disease (p < 0.03; OR = 10.0). There were no differences between RSV genotypes and the type of infection, i.e. single infection or co-infection (p = 0.5).
Table 4.
Genotypes of respiratory syncytial virus in outpatient and hospitalized infants, according to its clinical outcome.
| Clinical outcome | RSV genotypes |
|||
|---|---|---|---|---|
| Inpatients |
Outpatients |
|||
| NA1a | B7/B9 | NA1a | B7/B9 | |
| Severe | 10 | 2 | – | – |
| Moderate | 4 | 0 | – | 2 |
| Mild | 2 | 1 | 3 | 9 |
| Total | 19 | 14 | ||
NA1strains were more frequent in hospitalized infants (p < 0.001), and with severe disease (p = 0.01; OR = 10.0). Chi-square.
4.3. RSV viral load
Sixty-seven NPS were evaluated for viral load of RSV; 46 (68.7%) were from inpatients and 21 (31.3%) from outpatients. Inpatients’ median viral load was 417.300 copies/μl/sample (Standard Error (SE): 1341.2), compared to 133.200 copies/μl/sample (SE: 6569.3) from outpatients; (p = 0.7). Twenty-six samples corresponded to severe children, 20 to moderate and 21 to mild infants. Median viral load was 293.100 copies/μl/sample (SE: 2063.76) for severe patients, 286.200 copies/μl/sample (SE: 1277.04) for moderate disease and 360.600 copies/μl/sample (SE: 6577.52) for mild disease (p = 0.711). The median viral load of NA1 genotype was 963.000 copies/μl/sample (SE: 7435.02), while for B genotype was 33.525 copies/μl/sample (SE: 1571.546) (p = 0.04).
4.4. Rhinovirus genotypes
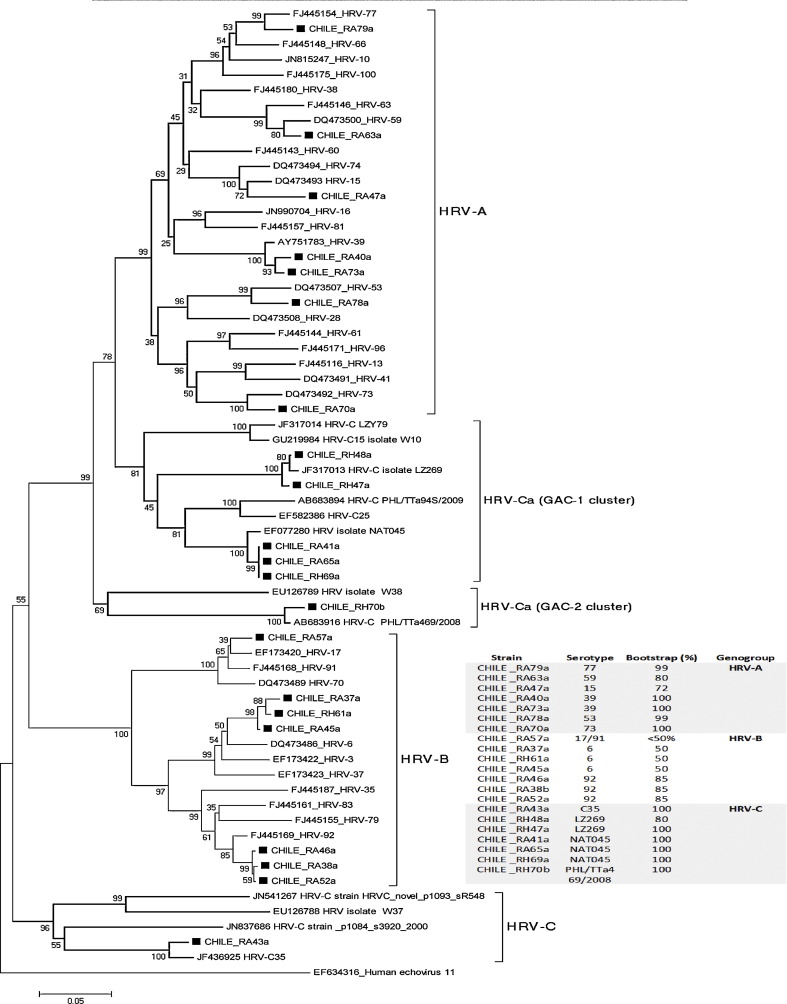
Fifty children were infected with HRV: 22 as a single infection and 28 as co-infection with RSV. We could typify 21/50 strains of HRV (42%); 13 outpatients and 8 hospitalized infants (Fig. 1 ). Seven strains belong to genogroup A, seven to the genogroup B and seven to genogroup C. There were no differences among genogroups of HRV, as a single infection or as co-infection whit RSV (p = 0.8), neither according to severity of illness.
Fig. 1.
Comparison of rhinovirus clinical isolates phylogeny using Neighbor-joining method. A total of 117 reference sequences obtained from Genbank were used for alignment and 43 of them are shown in the figure. Genbank accession numbers are indicated. Isolates were assigned to genogroup to which they clustered in the phylogenetic tree with a significant bootstrap value >50% [21]. HRV genogroups are indicated in the right side of each branch. HRV-Ca belongs to genogroup C but genetic similarity in the 5′NCR region forces clustering with HRV-A [17], [21], [41], [42], [43].
5. Discussion
During two consecutive winter seasons it was confirmed the high frequency of RSV infections in infants younger than 6 months, but also the high frequency of rhinovirus infections. The need for hospitalization of infants infected with RSV was significantly higher compared with those infected by HRV or co-infected with both viruses.
Unlike other authors, who observed that infants with ALRTI caused by HRV can be as serious as those infected with RSV [6], [8], [22], [23], our results indicate that RSV infection was significantly more severe than rhinovirus infection and also than co-infection with both RSV and HRV viruses. One possible explanation is that in our study patients were infants younger than 6 months of age, with a median of 2.6 months, which were attended in their first episode of respiratory infection. Similar results were found by Marguet et al. [9]. It is known that severe RSV infections mainly affect very young infants [2], [3], while severe HRV infections principally could affect children older than 6 months of age, or infants with previous morbidity [6], [8], [24].
Certain clinical conditions increase the risk of developing severe RSV disease. Likewise the impaired immune response, such as low counts of lymphocytes subpopulations and poor blood proinflammatory cytokine production, elicited by the virus plays a leading role in the severity of respiratory disease [1], [14], [25]. In recent years, new studies indicate that genetic factors linked to the host could have an important role in the phenotype of RSV disease [1], [26], [27], [28], [29]. Additionally, controversial results have been obtained regarding the link between genotypic variations of the virus and disease severity [18], [20], [30].
Previous studies have demonstrated the co-circulation of multiple RSV genotypes during annual outbreaks and the most prevalent strain depends on geographical and seasonal factors [31], [32]. No connection has been well established among circulating genotypes and the size of the epidemic, nor with their clinical impact, since results are contradictory [20], [32], [33]. In Chile, it has been detected more frequently epidemics with predominance of group A [34]. Previously, we found no differences between virus genogroup and clinical outcome. Until now, NA1 genotype had not been detected in Chile. This genotype was significantly more related to hospitalized infants and severe disease, especially during the winter season of 2010 and regardless of single RSV infection or a co-infection with HRV.
NA1 genotype was previously described and we believe that its introduction in Chile occurred in recent years [35], [36], [37]. This would explain why most of NA1 strains were found during 2010: 14 of 19 strains (73.7%) which also were significantly associated with severe disease. In fact, during 2011, although there was also circulation of NA1, B7 strain was the most prevalent, reducing significantly the severity of disease. Additionally, from 5 infants infected with NA1 in 2011, only one had a severe disease. So, we hypothesized that the emergence of new RSV strains may be associated with severe disease in infants when the general population is still not immune to these new genotypes.
Association between RSV loads and disease severity is still controversial. Although some authors have not founded correlation between severity of illness with titer of RSV [38], other studies have correlated significantly higher viral load with severe RSV bronchiolitis in infants [33], [39]. In our study, viral loads were similar between severe and mild patients except for the NA1 strain, whose loads were significantly higher than B7/B9 strains, probably because of the viral emergency in a susceptible population.
Although viral loads in respiratory samples could be affected by multiples variables such as time or amount of sample collected [30], [39], in our study sampling performed by trained personnel following a specific protocol, minimize the differences. Furthermore, these differences would not affect the observed results since would cause random effects rather than systematic bias.
Rhinovirus strains are grouped into three genogroups, A, B and C [17], [21], [40]. A few epidemiologic data exist on the relationship between HRV genogroups and illness severity. Kiang et al. found children hospitalized with severe respiratory illnesses, which were infected with genogroup C. In a prospective multicenter study, bronchiolitis in African American infants were more often associated with HRV than other children [6]. In our work, HRV infection was concentrated significantly in patients with mild disease, where strains were distributed homogeneously among the three known genogroups. The co-infection with both RSV and HRV viruses did not correlate with a more severe disease. The rate observed in HRV infection is similar to that reported by other authors. However, the clinical behavior of patients differs depending on the design of the studies. In this study, we have included very young infants, without previous morbidity and in their first episode of ALRTI. In our opinion, these characteristics influence the clinical behavior of studied patients.
In conclusion, severe RSV infections were in part influenced by the emergence of strain NA1. However, in our experience and according to other authors, we postulate that in infants younger than 6 months of age, the clinical severity of RSV infection is mainly due to the profile of immune response that is induced by the virus in each individual child [1], [14], [25]. In contrast, in very young infants infected by HRV, the relative immaturity of the immune system may avoid the induction of an intense inflammatory response, as it is seen in older infants or in atopic individuals throughout life. In the future, it will be necessary to compare meticulously the profile of the immune response on different cohorts with rhinovirus infection.
Funding
All funding was provided by the National Fund of Science and Technology; FONDECYT 1100477.
Competing interests
The authors declare that there are no conflicts of interest to disclose.
Ethical approval
Study protocol was approved by each of the Institutional Review Boards from the Roberto del Río Hospital, the Faculty of Medicine of the University of Chile and the National Fund of Science and Technology (FONDECYT 1100477). Informed and signed consent was obtained from the parents of all study participants as is required.
Acknowledgments
The authors thank to Mrs. Dina Silva, Rosa Corvalán, Mónica Peña and to Mr. Cristian Moreno for their technical assistance. Also we thank to Dr. Roberto Vidal for their help in phylogenetic analysis of rhinovirus and Dr. Bettina Von Dessauer and the nurses Mariel Bolvarán, Grisel Navarrete, Macarena Monsalve and Paulina Cifuentes who helped with patient's recruitment.
Contributor Information
Aldo Gaggero, Email: agaggero@med.uchile.cl.
Carmen E. Larrañaga, Email: clarrana@med.uchile.cl.
References
- 1.Collins P.L., Graham B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall C., Weinberg G., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorc J.J., Hall C.B. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 4.Avendaño L.F., Palomino M.A., Larrañaga C. Surveillance for respiratory syncytial virus in infants hospitalized for acute lower respiratory infection in Chile (1989–2000) J Clin Microbiol. 2003;41:4879–4882. doi: 10.1128/JCM.41.10.4879-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomino M.A., Larrañaga C., Villagra E., Camacho J., Avendaño L.F. Adenovirus and respiratory syncytial virus-adenovirus mixed acute lower respiratory infections in Chilean infants. Pediatr Infect Dis J. 2004;23:337–341. doi: 10.1097/00006454-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Mansbach J.M., Clark S., Christopher N.C., LoVecchio F., Kunz S., Acholonu U. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Pediatrics. 2008;121:680–688. doi: 10.1542/peds.2007-1418. [DOI] [PubMed] [Google Scholar]
- 7.Manoha C., Espinosa S., Aho S.L., Huet F., Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38(3):221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Korppi M., Kotaniemi-Syrjanen A., Waris M., Vainionpaa R., Reijonen T.M. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995–999. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 9.Marguet C., Lubrano M., Gueudin M., Le Roux P., Deschildre A., Forget C. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS ONE. 2009;4(2):e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander S.L., Busse W.W. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005;116:267–273. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Singh A.M., Moore P.E., Gern J.E., Lemanske R.F., Jr., Hartert T.V. Bronchiolitis to asthma. A review and call for studies of gene–virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 12.Jartti T., Lehtinen P., Vanto T., Hartiala J., Vuorinen T., Makela M. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 13.Mosser A.G., Brockman-Schneider R., Amineva S., Burchell L., Sedgwick J.B., Busse W.W. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 14.Larrañaga C., Ampuero S., Luchsinger V., Carrión F., Aguilar N., Morales P. Impaired immune response in severe human lower tract respiratory infection. Pediatr Infect Dis J. 2009;28(10):867–873. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Cane P., Pringle C. Molecular epidemiology of respiratory syncytial virus: rapid identification of subgroups A lineages. J Virol Methods. 1992;40:297–306. doi: 10.1016/0166-0934(92)90088-u. [DOI] [PubMed] [Google Scholar]
- 17.Kiang D., Kalra I., Yagi S., Louie J.K., Boushey H., Boothby J. Assay for 5′ noncoding region analysis of all human rhinovirus prototype strains. J Clin Microbiol. 2008;46:3736–3745. doi: 10.1128/JCM.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peret T., Hall C., Hammond G., Piedra P., Storch G.A., Sullender W.M. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venter M., Madhi S., Tiemessen C., Schoub B. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J Gen Virol. 2001;82:2117–2124. doi: 10.1099/0022-1317-82-9-2117. [DOI] [PubMed] [Google Scholar]
- 21.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krilov L., Pierik L., Keller E., Mahan K., Watson D., Hirsch M. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J Med Virol. 1986;19:345–352. doi: 10.1002/jmv.1890190407. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos N.G., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 24.Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 25.Welliver T.P., Garofalo R.P., Hosakote Y., Hintz K.H., Avendaño L., Sanchez K. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus us characterized by the absence of pulmonary cytotoxic lymphocytes responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ampuero S., Luchsinger V., Tapia L., Palomino M.A., Larrañaga C. SP-A1, SP-A2 and SP-D gene polymorphisms in severe acute respiratory syncyitial infection in chilean infants. Infect Genet Evol. 2011;11(6):1368–1377. doi: 10.1016/j.meegid.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Paulus S.C., Hirschfeld A.F., Victor R.E., Brunstein J., Thomas E., Turvey S.E. Common human toll-like receptor 4 polymorphisms – role in susceptibility to respiratory syncytial virus infection and functional immunological relevance. Clin Immunol. 2007;123:252–257. doi: 10.1016/j.clim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Lathi M., Löfgren J., Martilla R., Renko M., Klaavuniemi T., Haataja R. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hull J. Genetic susceptibility to RSV disease. In: Cane P., editor. vol. 14. Elsevier; Amsterdam: 2007. pp. 115–140. (Respiratory syncytial virus). [Google Scholar]
- 30.De Vincenzo J.P. Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr Res. 2004;56:914–917. doi: 10.1203/01.PDR.0000145255.86117.6A. [DOI] [PubMed] [Google Scholar]
- 31.Cane P. Molecular epidemiology of respiratory syncytial virus. Rev Med Virol. 2001;11:103–116. doi: 10.1002/rmv.305. [DOI] [PubMed] [Google Scholar]
- 32.Sato M., Satto R., Sakai T., Sano Y., Nishikawa M., Sasaki A. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J Clin Microbiol. 2005;43(1):36–40. doi: 10.1128/JCM.43.1.36-40.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fodha I., Vabret A., Ghedira L., Seboui H., Slaheddine Chouchane S. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol. 2007;79:1951–1958. doi: 10.1002/jmv.21026. [DOI] [PubMed] [Google Scholar]
- 34.Galiano M.C., Luchsinger V., Videla C.M., De Souza L., Puch S.S., Palomo C. Intragroup antigenic diversity of human respiratory syncytial virus (group A) isolated in Argentina and Chile. J Med Virol. 2005;77:311–316. doi: 10.1002/jmv.20456. [DOI] [PubMed] [Google Scholar]
- 35.Shobugawa Y., Saito R., Sano Y., Zaraket H., suzuki Y., Kumaki A. Emerging genotypes of human respiratory syncytial virus subgroup A among patients in Japan. J Clin Microbiol. 2009;47(8):2475–2482. doi: 10.1128/JCM.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sovero M., Garcia J., Kochel T., Laguna-Torres V.A., Gomez J., Chicaiza W. Circulating strains of human respiratory syncycytial virus in Central and South America. PLoS ONE. 2011;6(8):e22111. doi: 10.1371/journal.pone.0022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshaghi A., Duvvuri V., Lai R., Nadarajah J., Li A., Patel S. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario. A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE. 2012;7(3):e32807. doi: 10.1371/journal.pone.0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright P.F., Gruber C.W., Peters M., Reed G., Zhu Y., Robinson F. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002;185:1011–1018. doi: 10.1086/339822. [DOI] [PubMed] [Google Scholar]
- 39.El Saleeby C., Bush A., Harrison L., Aitken J., DeVincenzo J. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laine P., Savolainen C., Blomqvist S., Hovi T. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J Gen Virol. 2005;86:697–706. doi: 10.1099/vir.0.80445-0. [DOI] [PubMed] [Google Scholar]
- 41.Lysholm F., Wetterbom A., Lindau C., Darban H., Bjerkner A. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS ONE. 2012;7(2):e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simmonds P., McIntyre C., Savolainen-Kopra C., Tapparel C., Mackay I., Hovi T. Proposals for the classification of human rhinovirus species C into genotypically assigned types. J Gen Virol. 2010;91:2409–2419. doi: 10.1099/vir.0.023994-0. [DOI] [PubMed] [Google Scholar]
- 43.Fuji N., Suzuki A., Lupisan S., Sombrero L., Galang H. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS ONE. 2011;6(11):e27247. doi: 10.1371/journal.pone.0027247. [DOI] [PMC free article] [PubMed] [Google Scholar]