Fig. 1.
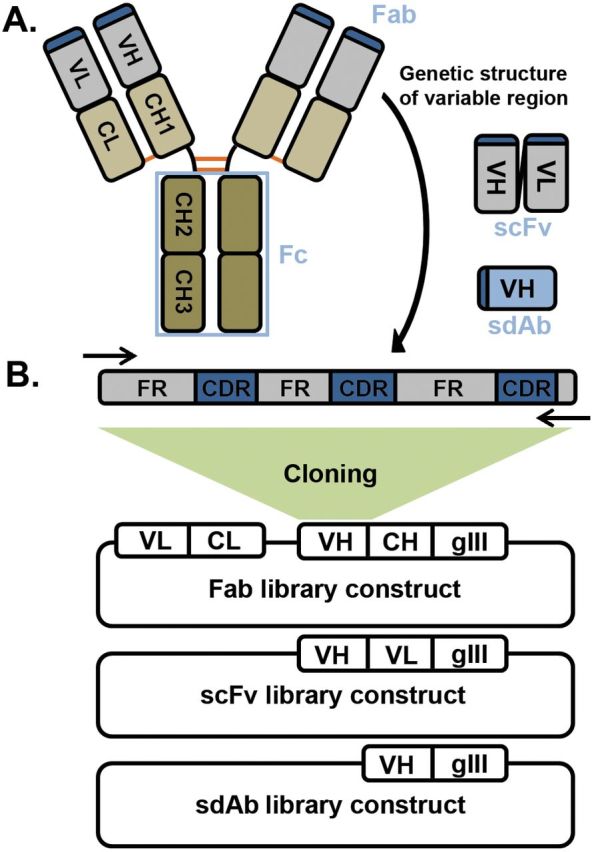
(A) Structure of a typical IgG molecule. Each antibody comprises two heavy and two light chains each of which have four and two immunoglobulin domains, respectively. The first domain is variable and determines specificity (VL and VH) while the second domain of the light chain (CL) and the second to fourth domains of the heavy (CH1-3) are constant across all antibodies of the same isotype. The light chain and first two domains of the heavy chain form the Fab, which is the portion expressed on the phage. The last two domains of the heavy chain form the Fc and are responsible for immune function through engagement of receptors on immune cells. Heavy and light chains are linked through a single disulfide bond (orange) between the CL and CH1 domains and the two heavy chains have multiple disulfide bonds at the hinge region between the CH1 and CH2. An scFv consists of just variable light and variable heavy domains joined by a flexible polypeptide linker while an sdAb as the name implies is only a single immunoglobulin (usually VH) domain which is sufficient for binding. (B) Variable domain genetic structure and construction of a natural phage display library. Each variable domain consists of three hypervariable CDRs interspersed between the more conserved FRs. The immunoglobulin domain folds such that the CDRs are brought together to form the antigen-binding surface at the tip of the Fab. Degenerate primers (arrows) are used to amplify the entire variable heavy and light chains (or alternatively variable and first constant domain) from a source of B cells and cloned in-frame with the phage coat protein (usually gene III) into E. coli to produce an Fab, scFv or sdAb library. The rest of the phage genome is supplied through replication defective helper phage to produce antibody-displaying phage.
