Abstract
Background
Viral diarrhea remains a major cause of childhood morbidity and mortality worldwide. Although rotavirus was extensively studied in China, few comprehensive studies of all viral agents related to diarrhea in children have been conducted.
Objectives
Our study was performed to investigate the role of enteric viruses in acute diarrhea in our country and to evaluate methods that could be used in routine diagnostics.
Study design
One hundred stool samples were collected from children under 5 years of age seeking medical care for acute diarrhea during the winter season 2000/2001 in Beijing Children's Hospital. All specimens were initially screened microscopically for leucocytes/red blood cells. Samples with negative results were analyzed for virus presence using commercial EIAs and/or in-house RT-PCRs.
Results
At least one viral agent was found in 67% of the specimens. The frequency of rotavirus, astrovirus, norovirus and enteric adenovirus was 59%, 8%, 6% and 2%, respectively. Dual infections were found in 9.0% (6/67) of the positive samples. The results from rotavirus and astrovirus EIAs were concordant with those of rotavirus and astrovirus RT-PCRs.
Conclusions
Enteric viruses play an important role in pediatric diarrhea during the winter season in China. A combination of microscopic examination of stool samples with specific EIA assays to detect virus antigen in stool specimens may be suitable for routine diagnostics.
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; EIA, enzyme immunoassay; EM, electron microscopy; RNA, ribonucleic acid; dNTP, deoxynucleotide triphosphate
Keywords: Diarrhea, Rotavirus, Astrovirus, Norovirus, Enteric adenovirus
1. Introduction
Diarrheal disease is a major cause of childhood morbidity and mortality, especially in developing countries (Bern et al., 1992). In the year 1990, it was estimated that 2.9 million deaths worldwide could be attributed to diarrheal disease (Murray and Lopez, 1997).
Several different groups of viruses have been shown to be responsible for the high incidence of acute viral diarrhea among children during their first few years of life. Four major categories of viruses are now recognized as clinically important including rotavirus, astrovirus, adenovirus and calicivirus (Wilhelmi et al., 2003). Rotavirus is the single most important etiological agent in severe dehydrating diarrhea. Each year, rotavirus causes approximately 111 million episodes of gastroenteritis requiring only home care, 25 million clinic visits, 2 million hospitalizations and 440,000 deaths in children under 5 years of age (Parashar et al., 2003). Astrovirus has only recently been recognized as a common cause of diarrhea in children. Currently, eight serotypes of astrovirus have been reported. They are responsible for 2.5% to 9% of cases hospitalized with diarrhea (Glass et al., 1996). A limited number of adenovirus strains have been associated with childhood diarrhea, types 40 and 41. Enteric adenovirus types 40/41 have been identified in up to 7.9% cases of diarrhea in children (Bon et al., 1999, Grimwood et al., 1995, Giordano et al., 2001, Qiao et al., 1999, Simpson et al., 2003). Human caliciviruses including norovirus and sapovirus are recognized as the main agents responsible for food-borne and nosocomial outbreaks of non-bacterial diarrhea. Recent studies investigating calicivirus in sporadic cases of gastroenteritis in children have concluded that caliciviruses comprise the second cause of viral diarrhea after rotavirus. (Bereciartu et al., 2002, Bon et al., 1999, Kirkwood and Bishop, 2001, Pang et al., 2000, Simpson et al., 2003, Subekti et al., 2002).
Defining the viral agents related to diarrhea will assist in providing an accurate estimate of disease burden within a community. This will allow for a proper assessment of the contribution of each virus to morbidity. The availability of accurate information on the etiology of viral diarrhea will also be useful when it comes to assessing the impact of vaccinations whenever they become available.
While rotavirus was extensively studied in our country, few studies have evaluated all the viral agents known to be related to diarrhea in young children. Our study was performed to determine the relative frequency of these viruses during a winter season in children seeking medical care for acute diarrhea in Beijing Children's Hospital. We also attempted to find simple diagnostic methods that could be used in routine diagnostic work in developing countries.
2. Materials and methods
2.1. Specimen collection
Stool samples from 100 children (62 boys and 38 girls) under 5 years of age (the median age was 9.5 months, range from 21 days to 60 months) attending the out-patient department of Beijing Children's Hospital, with diagnosis of acute diarrhea, were collected from December 2000 to March 2001. One stool specimen was collected from each patient. Diarrhea was defined as the occurrence of three or more unformed (loose or watery) stools within a 24 h period. Stool samples with no white or red blood cells by microscopic examination were selected. All specimens were stored at −70 °C until further analysis.
2.2. Microscopic examination
One drop of 0.9% normal saline was added to a glass slide and a small amount of feces was dispersed in it. Examination for tangible material including parasitic cysts and eggs, white and red blood cells was performed under a light microscope (Olympus, Japan). The microscopic examination was performed within one hour from collection of the stool sample.
2.3. Viral RNA extraction
Viral RNA from rotavirus was extracted manually from 140 μl 20% fecal suspension in phosphate-buffered saline using a QIAamp® Viral RNA Mini Kit (QIAGEN, Italy), following the manufacturer's recommendations. Viral RNA from calicivirus and astrovirus was extracted automatically from 200 μl 20% fecal suspension in phosphate-buffered saline using BioRobot M48 Workstation (QIAGEN).
2.4. Enteric adenovirus detection
Stool samples were tested for adenovirus types 40 and 41 using a commercial EIA kit (IDEIA™ Adenovirus Type 40/41, DAKO Ltd., Denmark) following the manufacturer's recommendations.
2.5. Rotavirus detection
Rotavirus antigen was detected using a commercial EIA kit (IDEIA™ Rotavirus, DAKO Ltd., Denmark) following the instructions of the manufacturer. Rotavirus was also detected by RT-PCR using the Beg9 and End9 pair of primers as previously described (Gouvea et al., 1991).
2.6. Astrovirus detection
Astrovirus antigen was detected using a commercial EIA kit (IDEIA™ Astrovirus, DAKO Ltd., Denmark) following manufacturer's instructions.
Astrovirus was also detected by RT-PCR using Ready-to-Go RT-PCR Beads (Amersham Biosciences AB, Sweden) with primers Mon 269 and Mon 270 (Noel et al., 1995). Briefly, 45 μl of DEPC-treated water containing 0.1 μM of primers Mon 269 and Mon 270 were added into each tube (one RT-PCR bead/tube) and incubated on ice until the bead dissolved. Five microliter of template RNA was added individually to each tube and the RT-PCR program was run in PE thermocycler as follows: 42 °C, 60 min; 94 °C, 3 min; 40 cycles of PCR (94 °C, 30 s; 50 °C, 30 s; 72 °C, 1 min) and a final 5 min extension at 72 °C. RT-PCR products were analyzed by agarose gel electrophoresis (1.5%) containing 0.5 μg/ml ethidium bromide followed by visualization under ultraviolet light.
2.7. Norovirus detection
Stool samples were analyzed for norovirus using RT-PCR with primers JV 12a and JV 13b (Vinjé and Koopmans, 1996). Briefly, 5 μl of template RNA was mixed with 1 μl of primer JV 13b (50 μM) and 3 μl of DEPC-treated water and heated at 94 °C for 2 min. After immediate cooling on ice for at least 2 min, the mixture was added to 6 μl of RT reaction cocktail containing 10 mM Tris–HCl, 50 mM KCl, 3 mM MgCl2, 1 mM each dNTP and 100 U of reverse transcriptase (Superscript, Invitrogen, USA), and incubated at 42 °C for 1 h, followed by 94 °C for 5 min. Five microliter cDNA was mixed with 45 μl of PCR reaction mixture containing 10 mM Tris–HCl, 75 mM KCl, 1.2 mM MgCl2, 0.1 mM of each dNTP, 0.3 μM of primer JV 12a and 2.5 U of Taq polymerase (Promega, USA). Forty amplification cycles were carried out (1 min at 94 °C, 1.5 min at 37 °C and 1 min at 74 °C), followed by a final extension at 74 °C for 7 min. The PCR products were analyzed on 1.5% agarose gel as described above.
3. Results
Among the 100 stool specimens from patients with diarrhea, 67 (67%) contained at least one of the four viruses examined for, whereas no virus could be detected in the 33 (33%) remaining samples. The frequency of each individual agent was as follows: rotavirus 59 (59%), astrovirus 8 (8%), norovirus 6 (6%) and enteric adenovirus 2 (2%). Dual or multiple infections were found in six out of the 67 positive samples (9.0%). All of these were combinations of rotavirus with other viruses. In one stool sample, three viral agents were identified and they were rotavirus, astrovirus and norovirus, respectively. Enteric adenoviruses were not found to co-infect with any other viruses (Fig. 1 ).
Fig. 1.
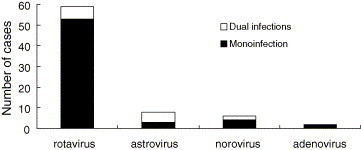
Mono- and mixed viral infections detected among young children with diarrhea in a winter epidemic in Beijing Children's Hospital. One patient was identified to be infected with rotavirus, astrovirus and norovirus.
For rotavirus and astrovirus, viruses were detected by both EIA and RT-PCR. Identical results were obtained with the two different methods.
4. Discussion
Although the importance of viral diarrhea as a prime cause of morbidity and mortality in developing countries is well recognized, to our knowledge few studies were conducted to evaluate the role of viral agents systematically in childhood diarrhea in China. Our study, performed during a winter season from December 2000 to March 2001 in Beijing, revealed that 67% of the outpatients under 5 years of age with non-leukocyte diarrhea were infected with at least one viral agent and of which 59% were infected with rotavirus. A prospective population-based surveillance in a rural county and a prospective hospital-based surveillance at three sentinel hospitals for rotavirus diarrhea among children under 5 years of age in China showed that rotavirus was identified in 51% of inpatients tested, 29% of outpatients, and 9.5% of children treated by village doctors (Fang et al., 2004). The high detection rate in our study may have at least two reasons. Firstly, our study was performed during a winter season, which is the peak season of viral diarrhea in temperate regions; secondly, the exclusion of samples with white or red blood cells before analysis for viral causes. Previous reports have found that examination of stool samples for leukocyte and blood is a rapid, reliable, and inexpensive way to differentiate between invasive bacterial and other causes of acute diarrhea (Alvarado, 1983, Siegel et al., 1987). A simple method as microscopic examination might still potentially be practical and economical in developing countries and regions where both bacterial and non-bacterial agents play important roles in acute diarrhea.
The comprehensive detection of the four viruses allowed us to observe a high percentage of dual infections among positive samples (9.0%), all of which were combinations of rotavirus and one or two of the other viruses. Whether a single virus is the main reason for the diarrheal illness or whether they potentiate each other still remains unclear. As previously reported, co-infections with astrovirus and rotavirus did not result in more severe symptoms than that with astrovirus as the only cause of infection (Herrmann et al., 1991). Mixed infections with viruses, bacteria and/or parasites may also occur but was in this study excluded only by microscopic examination investigating white and red blood cells suggestive of invasive bacterial infections and parasitic cysts and eggs.
Both EIA and RT-PCR were used in this study to detect rotavirus and astrovirus. This allowed us to compare the detection rate between those two methods. The results obtained from EIA matched very well with that from the RT-PCR for both viruses. RT-PCR assays are used more and more frequently in large scale investigations to understand the epidemiological features of viral diarrhea among children. This is done not only because it is at least by some investigators considered to be more sensitive than EM and EIA (Buesa et al., 1996, Mitchell et al., 1995), but also because it can be used to further genotype and compare the homogeneity of the circulating virus strains in different years and regions. However, our study indicates that EIA, which is easier to perform, faster and cheaper than RT-PCR, may in this setting be sufficiently sensitive and suitable for routine diagnostic work. Also other investigators have shown that although the RT-PCR may be more sensitive than EIA this may not be relevant in routine diagnostics since large amounts of viral particles, adequate for antigen detection by EIA, are shed in feces during a virus-induced diarrhea (Buesa et al., 1996).
Finally, our study shows a high frequency of rotavirus (59%) in children seeking medical care for diarrhea in a winter season in Beijing, China. In such a large developing country, introduction of a potent rotavirus vaccine would most likely be beneficial for children and a significant proportion of the diarrheal disease burden might be prevented in the near future. Other enteric viruses such as group C rotaviruses, sapoviruses, toroviruses, picobirnaviruses, kobuviruses and coronavirus were not been investigated in our study and further studies are needed to fully understand the etiology of viral diarrhea.
Acknowledgement
This study was kindly supported by a fellowship from the World Health Organization.
References
- Alvarado T. Faecal leukocyte in patients with infectious diarrhoea. Trans R Soc Trop Med Hyg. 1983;77(3):316–320. doi: 10.1016/0035-9203(83)90151-7. [DOI] [PubMed] [Google Scholar]
- Bereciartu A., Bok K., Gómez J. Identification of viral agents causing gastroenteritis among children in Buenos Aires, Argentina. J Clin Virol. 2002;25:197–203. doi: 10.1016/s1386-6532(02)00010-0. [DOI] [PubMed] [Google Scholar]
- Bern C., Martines J., de Zoysa I., Glass R.I. The magnitude of the global problem of diarrheal disease: a 10-year update. Bull World Health Org. 1992;70(6):705–714. [PMC free article] [PubMed] [Google Scholar]
- Bon F., Fascia P., Dauvergne M., Tenenbaum D., Planson H., Petion A.M. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol. 1999;37(9):3055–3058. doi: 10.1128/jcm.37.9.3055-3058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesa J., Colomina J., Raga J., Villanueva A., Prat J. Evaluation of reverse transcription and polymerase chain reaction (RT/PCR) for the detection of rotaviruses: applications of the assay. Res Virol. 1996;147(6):353–361. doi: 10.1016/S0923-2516(97)85127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z.Y., Zhang L.J., Zhang Q., Xie H.P., Sun L.W., Tang J.Y. Proceedings of the Sixth International Rotarirus Symposium on Rotavirus Epidemiologic Trends in Chinese Mainland, 2003 (Abstract); Mexico City, July 7–9; 2004. [Google Scholar]
- Giordano M.O., Ferreyra L.J., Isa M.B., Martinez L.C., Yudowsky S.I., Nates S.V. The epidemiology of acute gastroenteritis in hospitalized children in Cordoba city Argentina: an insight of disease burden. Rev Inst Med Trop S Paulo. 2001;43(4):193–197. doi: 10.1590/s0036-46652001000400003. [DOI] [PubMed] [Google Scholar]
- Glass R.I., Noel J., Mitchell D., Herrman J.E., Blacklow N.R., Pickering L.K., Dennehy P., Ruiz-Palacios G., de Guerrero M.L., Monroe S.S. The changing epidemiology of astrovirus-associated gastoenteritis: a review. Arch Virol Suppl. 1996;12:287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Glass R.I., Woods P., Taniguchi K., Clark H.F., Forrester B. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1991;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., Carzino R., Barnes G.L., Bishop R.F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J Clin Microbiol. 1995;33(1):131–136. doi: 10.1128/jcm.33.1.131-136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J.E., Taylor D.N., Echeverria P., Blacklow N.R. Astrovirus as a cause of gastroenteritis in children. New Engl J Med. 1991;324(25):1757–1760. doi: 10.1056/NEJM199106203242501. [DOI] [PubMed] [Google Scholar]
- Kirkwood C.D., Bishop R.F. Molecular detection of human calicivirus in young children hospitalized with acute gastroenteritis in Melbourne, Australia, during 1999. J Clin Microbiol. 2001;39(7):2722–2724. doi: 10.1128/JCM.39.7.2722-2724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.K., Monroe S.S., Jiang Xi, Matson D.O., Glass R.I., Pickering L.K. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase-polymerase chain reaction. J Infect Dis. 1995;172:1437–1444. doi: 10.1093/infdis/172.6.1437. [DOI] [PubMed] [Google Scholar]
- Murray C.J.L., Lopez A.D. Mortality by causes for eight regions of the world, global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Noel J.S., Lee T.W., Kurtz J.B., Glass R.I., Monroe S.S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol. 1995;33(4):797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X.L., Honma S., Nakata S. Human clicivirus in acute gastroenteritis of young children in the community. J Infect Dis. 2000;181(Suppl. 2):288–294. doi: 10.1086/315590. [DOI] [PubMed] [Google Scholar]
- Parashar U.D., Hummelman E.G., Bresee J.S., Miller M.A., Glass R.I. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H.P., Nilsson M., Abreu E.R., Hedlund K.O., Johansen K., Zaori G. Viral diarrhea in children in Beijing. Chin J Med Vriol. 1999;57:390–396. [PubMed] [Google Scholar]
- Siegel D., Cohen P.T., Neighbor M., Larkin H., Newman M., Yajko D. Predictive value of stool examination in acute diarrhea. Arch Pathol Lab Med. 1987;111:715–718. [PubMed] [Google Scholar]
- Simpson R., Aliyu S., Iturriza-Gómara M., Desselberger U., Gray J. Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J Med Virol. 2003;70:258–262. doi: 10.1002/jmv.10386. [DOI] [PubMed] [Google Scholar]
- Subekti D., Lesmana M., Tjaniaki P., Safari N., Frazier E., Simanjuntak C., Komalarini S., Taslim J., Campbell J.R., Oyofo B.A. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol Med Microbiol. 2002;33:27–33. doi: 10.1111/j.1574-695X.2002.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Vinjé J., Koopmans M.P. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis. 1996;174(3):610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- Wilhelmi I., Roman E., Sánchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect. 2003;9(4):247–262. doi: 10.1046/j.1469-0691.2003.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


