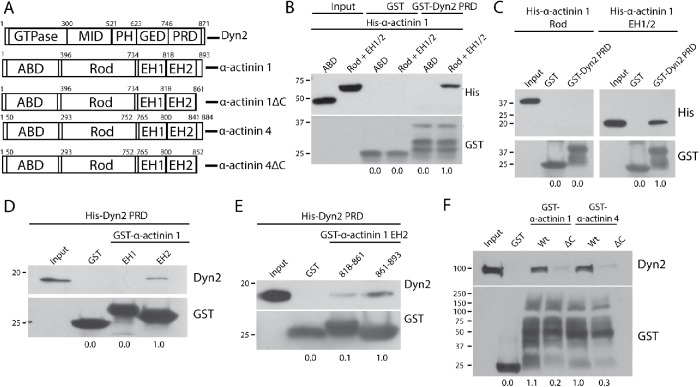
FIGURE 3:
The C-terminal tails of α-actinin 1 and 4 are responsible for binding to Dyn2. (A) Schematic diagrams showing the domain structure of Dyn2 and both α-actinin isoforms. Also depicted are the α-actinin 1ΔC (rat α-actinin 1: Δ862–893; human α-actinin 1: Δ861–892) and α-actinin 4ΔC (human α-actinin 4: Δ853–884) forms that cannot bind Dyn2. (B) GST pull down of GST-Dyn2 PRD was used to test binding to different regions in α-actinin 1. His–α-actinin 1 proteins representing the ABD (amino acids 1–395) and the rod + EH 1/2 domains (amino acids 396–893) were generated. GST was used as a control and showed no binding interaction. n = 3 independent experiments. (C) GST pull down of GST-Dyn2 PRD was used to test whether the Rod domain (amino acids 396–733) or the EH1/2 domain (amino acids 734–893) of α-actinin 1 is responsible for binding to Dyn2. n = 3 independent experiments. (D) Proteins representing the EH1 domain (amino acids 734–817) and EH2 domain (amino acids 818–893) were used to test binding to the Dyn2 PR. n = 2 independent experiments. (E) The α-actinin 1 EH2 domain was further separated into an N-terminal half (amino acids 818–861) and a C-terminal half (862–893) to further characterize the Dyn2-binding region. n = 3 independent experiments. (F) GST–α-actinin proteins were generated with deletions of the identified Dyn2-binding region in human α-actinin 1 (amino acids 861–892), and the predicted Dyn2-binding region in human α-actinin 4 (amino acids 853–884) based on sequence similarity to α-actinin 1. The GST fusion proteins were used in pull-down assays with lysates from DanG cells. n = 3 independent experiments. For B–F, relative average binding values are listed below each condition.