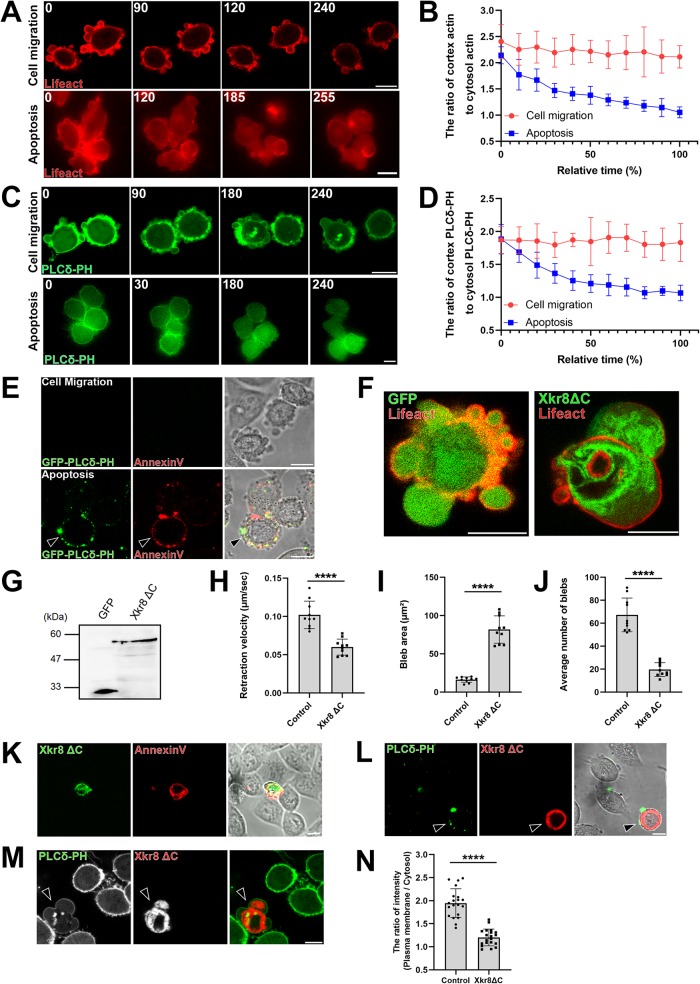
FIGURE 5:
Bleb expansion is triggered by loss of plasma membrane phospholipid asymmetry. (A, C) Membrane blebbing of migrating (top panels) and apoptotic (bottom panels) DLD1 cells expressing either Lifeact–RFP (A) or PLCδ-PH-GFP (C). Apoptosis was induced by simultaneous treatment with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 3 h. Times indicated are relative to an arbitrary starting point for migratory cells and to the first image after treatment for apoptotic cells (min). Results shown are representative of five independent experiments. Scale bar, 10 μm. (B, D) Quantification of experiments depicted in A and C. Fluorescence intensities of either Lifeact-RFP (B) or PLCδ-PH-GFP (D) were measured at the cell cortex and the cytosol and their ratio were plotted against time. The SD was calculated based on the values from N = 5 independent experiments. (E) PtdIns(4,5)P2 and PS exposure to the outer leaflet of the plasma membrane during apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 4 h. Migrating and late-phase apoptotic cells were stained with purified GFP-tagged PLCδ-PH protein and AnnexinV-Cy3. Arrowhead shows the apoptotic cell stained with PLCδ-PH and AnnexinV. Of note, the attachment of lipid binding proteins such as PLCδ-PH or Annexin V to the outer leaflet of plasma membranes inhibits bleb expansion. Therefore, the morphology of blebs of cells stained with these proteins are different from that of untreated cells. Result shown is representative of three independent experiments. Scale bar, 10 μm. (F) Membrane blebbing of DLD1 cells transfected with GFP tag only and Lifeact- RFP (left) and membrane blebbing of DLD1 cells transfected with GFP-tagged Xkr8ΔC, a constitutively active form of Xkr8, and Lifeact-RFP (right). Result shown is representative of three independent experiments. Scale bar, 10 μm. (G) Total cell lysates of DLD1 cells expressing GFP tag only and GFP-tagged Xkr8ΔC separated by SDS–PAGE and immunoblotted with an anti-GFP mAb. (H) The retraction velocities of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP were quantified. The SD was calculated based on the values from N = 10 independent blebs. ****P < 0.0001 (Student’s t test). Expression of Xkr8ΔC-GFP led to the reduction in the speed of the retraction phase of membrane blebs. (I) The sizes of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP were quantified. The SD was calculated based on the values from N = 10 independent blebs. ****P < 0.0001 (Student’s t test). Expression of Xkr8ΔC-GFP led to the enlargement of bleb size. (J) The frequencies of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP during 10 min were quantified. The SD was calculated based on the values from N = 10 independent cells. ****P < 0.0001 (Student’s t test). (K) PS exposure in Xkr8ΔC expressing DLD1 cell. Cells were stained with AnnexinV-Cy3. Result shown is representative of three independent experiments. Scale bar, 10 μm. (L) DLD1 cells expressing RFP-tagged Xkr8ΔC were stained with purified GFP-tagged PLCδ-PH protein to detect PtdIns(4,5)P2 exposure to the outer leaflet of the plasma membrane (arrowheads). Result shown is representative of five independent experiments. Scale bar, 10 μm. (M) PtdIns(4,5)P2 localization at the inner leaflet of the plasma membrane was observed by stably expressing PLCδ-PH-GFP in DLD1 cells. Arrowhead indicates the Xkr8ΔC-RFP-coexpressing cell. Result shown is representative of five independent experiments. Scale bar, 10 μm. (N) The fluorescence intensity of the PLCδ-PH-GFP (inner leaflet) signal was quantified in wild-type DLD1 cells and Xkr8ΔC-RFP-expressing DLD1 cells. The SD was calculated based on the values from N = 20 independent cells. ****P < 0.0001 (Student’s t test).