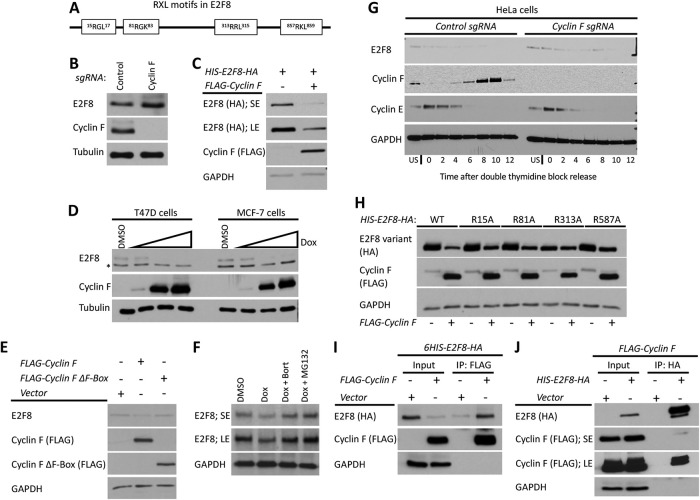
FIGURE 8:
Cyclin F mediates the degradation of E2F8 in G2-phase. (A) Schematic depiction of the RxL motifs in E2F8. (B) The abundance of E2F8 in control Cyclin F KO HeLa cells was analyzed by Western blot. (C) HEK293T cells were transiently transfected with HIS-E2F8-HA with and without FLAG-Cyclin F. Cells were analyzed by Western blot 48 h posttransfection. SE, short exposure; LE, long exposure. (D) MCF7 and T47D were engineered to express doxycycline (Dox) inducible Cyclin F. Cells were treated with doxycycline at increasing concentrations for 48 h and then endogenous E2F8 was analyzed by Western blot. (E) HEK293T cells were transiently transfected with FLAG-Cyclin F or its ΔF-box variant and analyzed by Western blot 48 h posttransfection. (F) MCF7 cells were treated with doxycycline to induce expression of Cyclin F. Eight hours prior to harvesting for Western blot, cells were treated with either of two proteasome inhibitors, MG132 and bortezomib. Endogenous E2F8 was analyzed by Western blot. (G) Control and Cyclin F KO HeLa cells were synchronized at the G1-S boundary by a double thymidine block. Following release from the second thymidine block, samples were collected for Western blot analysis at the indicated time points. (H) HEK293T cells were transfect with wild-type or mutant versions of HIS-E2F8-HA harboring alanine substitutions at the indicated RxL motifs shown in A. Their response to ectopic coexpression was analyzed by Western blot 48 h after transfection. (I, J) HIS-E2F8-HA and FLAG-Cyclin F were cotransfected into HEK293T cells. Cell lysates were subjected to co-IP with either anti-HA or anti-FLAG antibodies.