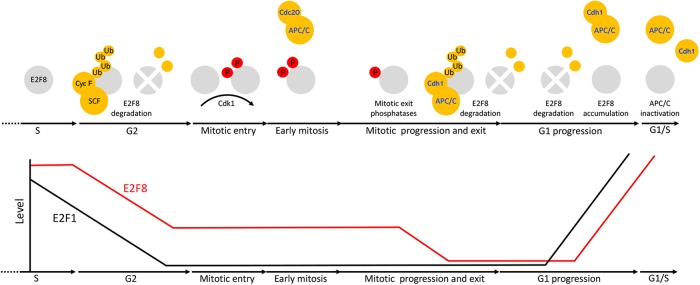
FIGURE 9:
Multiple mechanisms coordinate the dynamics of E2F8 in cycling mammalian cells. At the transcriptional level, E2F8 is primarily regulated by E2F1 via a negative feedback mechanism. Posttranslationally, E2F8 is controlled by temporal proteolysis orchestrated by multiple pathways. E2F8 peaks in S-phase. During G2-phase, E2F8 protein is down-regulated by SCFCyclin F activity. Although low-leveled, E2F8 proteolysis during early mitosis, while APC/CCdc20 is active, is inefficient. E2F8 is phosphorylated in mitosis by Cdk1. This phosphorylation has a stabilizing effect on the protein. During mitotic exit, Cdk1 is inactivated and both E2F8 and Cdh1 are dephosphorylated. This dual molecular switch initiates both the assembly of APC/CCdh1 and its ability to ubiquitinate E2F8. The levels of E2F8 remain minimal through G1 as long as APC/CCdh1 is fully active. During late G1, APC/CCdh1 activity weakens by an autonomous mechanism. The enhanced sensitivity of E2F8 to suboptimal APC/CCdh1 activity effectively stabilizes the protein while Securin and perhaps other APC/C targets are still degraded. Because E2F1 is already present, the negative feedback circuitry between E2F1 and E2F8 can be formed already in late G1 in ensuring a safe transition into S-phase.