Figure 1.
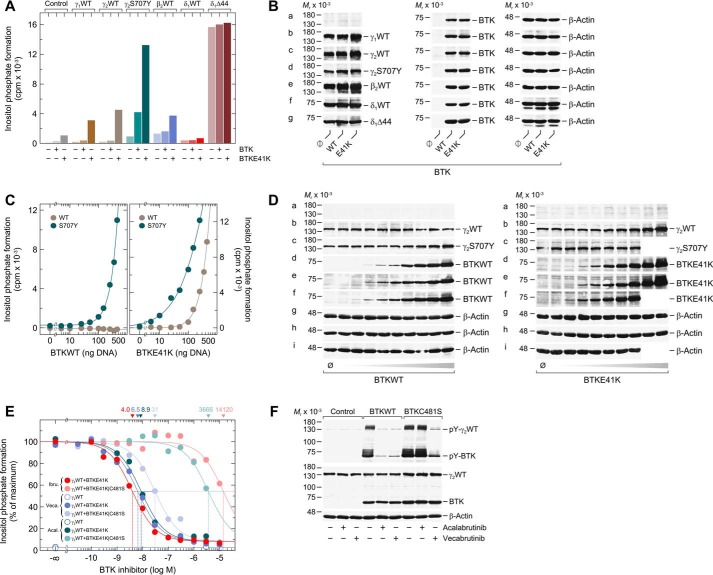
Analysis of the functional reconstitution of PLCγ2 and BTK. A, COS-7 cells were transfected as indicated with 250 ng/well of either empty vector (Control) or 150 ng/well of vector encoding either WT PLCγ1 (γ1WT), WT PLCγ2 (γ2WT), WT PLCβ2 (β2WT), or WT PLCδ1 (δ1WT), 50 ng/well of vector encoding PLCγ2S707Y (γ2S707Y) or PLCδ1Δ44 (δ1Δ44), and 100 ng/well of vector encoding either WT BTK (BTKWT) or a constitutively-active version of BTK (BTKE41K). B, cells from one well each functionally analyzed in A were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ1, WT PLCγ2, PLCγ2S707Y, WT PLCδ1, and PLCδ1Δ44 (a–d and f–g), antibody reactive against PLCβ2 (e), antibody reactive against BTK (a–g), or antibody reactive against β-actin (a–g). C, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) or PLCγ2S707Y (γ2S707Y) and increasing amounts (0–450 ng/well) of vector encoding WT BTK (BTKWT) (left panel) or BTKE41K (right panel). A and C, 24 h after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol, and inositol phosphate formation was then determined. D, cells from one well each functionally analyzed in C were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ2 and PLCγ2S707Y (a–c), antibody reactive against BTK (d–f), or antibody reactive against β-actin (g–i). E, COS-7 cells were transfected as indicated with 150 ng/well of vector encoding WT PLCγ2 (γ2WT) and 100 ng/well of vector encoding BTKE41K or BTKE41K/C481S. Twenty four hours after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol and either solvent (0.1% (v/v) DMSO) or increasing amounts of ibrutinib (0.1 nm to 10 μm), vecabrutinib (0.1 nm to 10 μm), or acalabrutinib (1 nm to 3 μm). Inositol phosphate formation was then determined. The results were normalized to percent of maximal inositol phosphate formation, which was 10,012, 9747, and 15,178 cpm for ibrutinib, acalabrutinib, and vecabrutinib, respectively. The IC50 values of ibrutinib, acalabrutinib, and vecabrutinib for the inhibition of BTKE41K and BTKE41K/C481S obtained by nonlinear curve fitting are shown above the graph in nm. F, COS-7 cells were transfected as indicated with 1.33 μg/well of vector encoding WT PLCγ2 (γ2WT) and 0.66 μg/well of empty vector (Control) or vector encoding BTKWT or BTKC481S. Twenty four hours after transfection, the cells were incubated for 18 h, as indicated with either solvent (0.1% (v/v) DMSO), acalabrutinib (1 μm), or vecabrutinib (1 μm). Cells were lysed, and immunoblotting was performed as indicated using an antibody reactive against the c-Myc epitope present on WT PLCγ2, antibody reactive against BTK, antibody reactive against β-actin, antibody reactive against phosphorylated tyrosine on PLCγ2 at position 759 (pY-PLCγ2), and antibody reactive against phosphorylated tyrosine on BTK at position 223 (pY-BTK). A, C, E, and F show representative results from three independent experiments each as mean values of three technical replicates.